12 Other Adjunctive Drugs for Coronary Intervention
β-Blockers, Calcium Channel Blockers, and Angiotensin-Converting Enzyme Inhibitors
 β-Adrenergic Receptors
β-Adrenergic Receptors
β-receptors belong to a well-characterized family of receptors known as G protein–coupled receptors.1 The pathway involves the binding of an agonist (e.g., catecholamines for β-receptors) to an extracellular receptor. Receptor activation causes a coupled G protein to stimulate adenylyl cyclase, which increases intracellular concentrations of cyclic adenosine monophosphate (cAMP). cAMP activates several AMP-dependent protein kinases, which phosphorylate other proteins, resulting in a cellular response. The cellular response for β-receptors differs according to three major subtypes: β1, β2, and β3. Whereas stimulation of β2 receptors causes bronchodilation and peripheral vasodilation, stimulation of β1 receptors predominantly affects the heart, increasing contractility and heart rate as well as lipolysis. The β3 receptor increases heat production in brown adipose tissue, and increases lipolysis in both brown and white adipose tissue.2,3 Interestingly, the β3 receptor may play a role in obesity and insulin resistance.3
β-Adrenergic Receptor Blockers
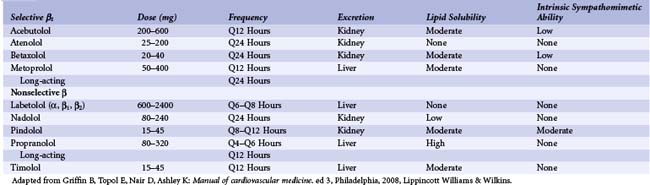
β-blockers act by directly competing with binding of catecholamines to β-adrenergic receptors. These agents differ in their selectivity, lipid-solubility, metabolism, and partial-agonist ability (intrinsic sympathomimetic ability [ISA]) (Table 12-1).53 Although some data suggest that these differences might impact efficacy in certain conditions (e.g., chronic congestive heart failure [CHF]), these differences mainly influence side effects, contraindications, and the frequency of dosing. For example, nonselective agents may increase bronchospasm in patients with asthma. Lipophilic agents may have more central nervous system (CNS) effects such as sedation and depression. Type of metabolism will affect plasma half-life in patients with renal or hepatic insufficiency. β-blockers with ISA slow heart rate less than β-blockers without ISA; also, β-blockers with ISA are less likely to decrease high-density lipoprotein (HDL) or increase triglycerides. Despite these pharmacokinetic differences, efficacy in CAD arises primarily from β1-receptor antagonism. In acute MI, for example, the catecholamine storm decreases the fibrillation threshold, increases myocardial oxygen consumption, and promotes myocardial necrosis. By decreasing heart rate and contractility, blockade of the β1 receptor lowers myocardial stress, which decreases necrosis. β-blockade also raises the fibrillation threshold. By antagonizing lipolysis, β-blockers reduce the concentrations of free fatty acids, causing a greater use of glucose and a lesser use of oxygen. β-blockers, in particular carvedilol, may also inhibit platelet aggregation, but this is still being debated; the mechanism could be membrane interaction instead of β-receptor antagonism.4 In light of these effects, it is not surprising that numerous clinical trials have demonstrated the benefits of β-blockers in acute coronary syndrome (ACS). Of course, prudence is still required in the use of β-blockers, as they decrease inotropy and slow atrioventricular (AV) conduction, which can be harmful in certain subgroups.
Unstable Angina Pectoris
Because of β-blockers’ potent effects in reducing myocardial oxygen demand, treating unstable angina with β-blockers has much intuitive appeal. A few small randomized trials have supported this. Gottlieb and colleagues randomized 81 patients with unstable angina to 4 weeks of propranolol or placebo.5 All patients received calcium channel blockers, nitrates, or both. Although the incidence of death, MI, or need for urgent coronary artery bypass grafting (CABG) did not differ between groups, propranolol significantly reduced the frequency and severity of recurrent ischemia. In the Holland Interuniversity Nifedipine and Metoprolol Trial (HINT), 338 patients with unstable angina not pretreated with a β-blocker randomly received nifedipine alone, metoprolol alone, or nifedipine and metoprolol.6 The odds ratios (ORs) for recurrent ischemia or MI by 48 hours were 1.15 (95% confidence interval [CI] 0.83–1.64) for nifedipine, 0.76 (95% CI 0.49–1.16) for metoprolol, and 0.80 (95% CI 0.53–1.19) for both; not surprisingly, the small numbers limited power of the study, and these differences were not statistically significant. Hohnloser and associates examined the effects of esmolol, a short-acting (half-life 9 minutes) intravenous β-blocker, in a randomized, placebo-controlled trial of 113 patients. The investigators increased esmolol until they reduced the double-product by approximately 25%; thereafter, the esmolol infusion continued for up to 72 hours.7 Acute MI or urgent revascularization occurred in 9 patients treated with placebo compared with 3 patients treated with esmolol (P = 0.06). In a more recent randomized trial, Brunner and colleagues randomized 116 patients with unstable angina to placebo or carvedilol at 25 mg twice a day.8 Patients received 48-hour Holter monitoring to document ischemia. Carvedilol reduced ischemic time by 75% (204 vs. 49 minutes, P < 0.05) with a 66% reduction in number of ischemic episodes (24 vs. 8, P < 0.05). Some retrospective data from recent studies also demonstrate the benefit of β-blockers in unstable angina. Ellis and associates pooled data from five randomized trials of abciximab during percutaneous coronary intervention (PCI)—the EPIC, EPILOG, EPISTENT, CAPTURE, and RAPPORT trials.9 Except for RAPPORT, which had patients with STEMI, the other four trials included patients with unstable angina or NSTEMI. All-cause mortality by 30 days occurred in 0.6% of patients receiving β-blockers compared with 2% in patients not receiving β-blockers. After adjusting for baseline characteristics and propensity score to receive β-blockers, β-blockers remained predictive of lower mortality (hazard ratio [HR] 0.25; 95% CI 0.1–0.57, P = 0.001). This mortality difference persisted at 6 months (1.7% vs. 3.7%, adjusted HR 0.53; 95% CI 0.29–0.94, P = 0.03). Among patients with unstable angina, β-blockers reduced mortality at 3 months (1.6%–0.6%, P = 0.029) and at 6 months (3.1%–1.4%, P = 0.009). Similarly, investigators found the mortality benefit of β-blockers in patients enrolled in the American College of Cardiology/American Heart Association Guidelines (CRUSADE) initiative named “Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation?”.10 In 72,054 patients with NSTEMI at 509 U.S. hospitals from 2001 to 2004, acute β-blocker use was associated with a lower hospital mortality (adjusted OR 0.66; 95% CI 0.60–0.72, P < 0.01). Importantly, nearly all patient subgroups benefited, including patients 80 years or older.
Percutaneous Coronary Intervention
Although trials have evaluated adjunctive β-blockade in patients with unstable angina or MI undergoing PCI, less data exist for effect of β-blockade as a specific adjunct to PCI. In fact, most data for adjunctive benefit arise from nonrandomized registries. Sharma and colleagues evaluated 1675 consecutive patients undergoing PCI.11 None of the patients had had MI before the PCI. The authors did not specify how many patients presented with unstable angina. Creatine kinase–muscle brain (CK-MB) elevation occurred in 13.2% of patients on β-blockers before the procedure compared with 22.1% of patients not on β-blockers (P < 0.001). On multivariate analysis, β-blockers remained an independent predictor of lower CK-MB release. Over a mean 15 months follow-up, patients on pre-procedural β-blockers had a mortality of 0.8% compared with 2% for patients not on pre-procedural β-blockers (P = 0.04). Chan and colleagues evaluated 4553 consecutive patients without acute or recent MI who underwent PCI, on the basis of whether or not they had been treated with β-blockers at the time of the PCI.12 Of these patients, 2056 (45%) were on β-blockers at the time of the intervention. Mortality was lower among patients on β-blockers at 30 days (1.3% vs. 0.8%, P = 0.13) and at 1 year (6% vs. 3.9%, P = 0.0014). After adjusting for differences in the baseline characteristics by propensity analysis, β-blocker therapy remained independently predictive of 1-year survival (HR 0.63; 95% CI 0.46–0.87, P = 0.0054). Along with these mortality data, other data suggest the benefit of β-blockers on re-stenosis. Jackson and colleagues followed up 4840 patients undergoing PCI on the basis of whether or not they received β-blockers on discharge.13 Patients treated with β-blockers had a 5-year clinical re-stenosis rate of 12% versus 14% (adjusted OR 0.83, P = 0.046). These data, however, are controversial because a small randomized trial of adjunctive carvedilol failed to show any reduction in re-stenosis in patients undergoing atherectomy.14 In another small randomized trial, Wang and colleagues examined the effect of intracoronary (IC) propranolol during PCI.15 In this trial, investigators randomized 150 patients undergoing PCI to placebo or propranolol (15 µg/kg) injected into the distal coronary artery via a balloon catheter positioned across the stenosis. CK-MB elevation occurred in 17% of the propranolol group compared with 36% of the placebo group (P = 0.01). The incidence of death, MI, or urgent revascularization by 30 days occurred in 18% of the propranolol group compared with 40% of the placebo group (P = 0.004). The relative risk of MI did not differ between the group on prior β-blocker therapy and the group not on prior therapy. Uretsky and colleagues also examined the effect of IC β-blockers during PCI by randomizing 400 patients to IC propranolol or placebo combined with systemic eptifibatide.16 At 1 year, the composite endpoint of death, postprocedural MI, urgent target lesion revascularization, or MI after hospitalization occurred in 21.5% of the propranolol group and 32.5% of the placebo group (P < 0.01); this was driven primarily by lower peri-procedural MI (12.5% propranolol vs. 21.5% placebo; RR reduction 0.43; 95% CI 0.08–0.65, P = 0.018).
Acute Myocardial Infarction
Early Trials
The data for β-blockade in acute MI come from 26 small trials and two large trials—the Metoprolol in Acute Myocardial Infarction (MIAMI) trial and the First International Study of Infarct Survival (ISIS-1) trial.17,18
Recent Data—The COMMIT Trial
Given that most data for β-blockade in acute MI are several decades old, benefit for β-blockade in the current era—with aggressive use of anti-platelet therapy, thrombolysis or primary angioplasty, statin therapy, and anti–aldosterone therapy—has remained uncertain, and physicians have hoped for trials with modern background therapy to assess the true value of β-blockade. This uncertainty has remained relevant because of persistent fears that β-blockers may exacerbate the condition of some patients with acute MI, particularly those with signs and symptoms of CHF. Fortunately, new data have come recently from the large-scale randomized CLOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).19 In fact, COMMIT was not only a trial performed in the modern reperfusion era, but it also was the largest trial ever investigating β-blockers in acute MI. As such, it is exceptionally important to have an in-depth understanding of the trial and its implications for patient management. COMMIT (also known as the Second Chinese Cardiac Study—CCS-2) was a placebo-controlled randomized trial with a two-by-two factorial design, randomizing patients with acute MI to metoprolol or placebo as well as to clopidogrel or placebo, with a background therapy of aspirin, anticoagulant therapy (mostly unfractionated heparin), and thrombolysis.
Patient Selection
The scale of the trial was impressive. Between August 1999 and February 2005, COMMIT enrolled 45,852 patients in 1250 Chinese hospitals. Inclusion criteria included left bundle branch block (presumably new), ST elevation, or ST depression within 24-hours of ischemic symptoms. Exclusion criteria included patients scheduled for primary PCI (because of combined aspirin and clopidogrel use that would interfere with the other study arm) or conditions considered high risk for β-blocker therapy—systolic blood pressure <100 mm Hg or heart rate <50 beats per minute (beats/min), heart block, or cardiogenic shock. Interestingly, moderate heart failure (Killip class II or III) was not a contraindication, unlike in trials such as MIAMI.18
Results
Not surprisingly, the large sample size of COMMIT ensured that baseline characteristics between groups were similar (Fig. 12-1). The mean age was 61 years with 26% older than 70 years, and 72% were male. ST elevation occurred in 87%, with left bundle branch block in 6% and ST depression in 7%. Time from symptom onset to treatment was evenly distributed over 24 hours, with approximately one third of patients treated within 6 hours, one third within 6 to 13 hours, and one third within 13 to 24 hours. Although most patients had no signs or symptoms of CHF, a sizable percentage (24%) had Killip class II or III on presentation. Again, this contrasts with the early β-blocker trials that enrolled lower-risk patients with no evidence of CHF. In COMMIT, 54% of patients received thrombolysis, with most of them receiving urokinase. Of those presenting within 12 hours, 68% received thrombolysis; it is unknown how many of the remaining patients did not receive thrombolytics because of clear contraindications. Notably, slightly fewer metoprolol-treated patients received ACE inhibitors (67.2% vs. 69.3%, P < 0.0001). The primary composite outcome of death, re-infarction, or cardiac arrest did not differ between metoprolol-treated patients (9.4%) and placebo-treated patients (9.9%; OR 0.96%; 95% CI 0.90–1.01, P = 0.10) (Fig. 12-2). Similarly, the co-primary outcome of death did not differ between the metoprolol (7.7%) and placebo (7.8%) groups (see Figure 12-1). Given the prior clinical data supporting early β-blockade in acute MI, reasons for these counterintuitive results require a careful dissection; the questions, in particular, are: Did β-blockade reduce any particular clinical events? Did β-blockade benefit some patients much more than others? β-blockade significantly reduced any re-infarction (2% for metoprolol vs. 2.5% for placebo, P = 0.001) and risk of ventricular fibrillation (2.5% vs. 3.0%, P = 0.001). The treatment, however, increased the risk of cardiogenic shock by 30% (5.0% vs. 3.9%, P < 0.0001). Therefore, although β-blockade significantly reduced arrhythmic death by 22% (1.7% for metoprolol vs. 2.2% for placebo, P = 0.0002), β-blockade significantly increased death secondary to cardiogenic shock by 29% (2.2% vs. 1.7%, P
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree