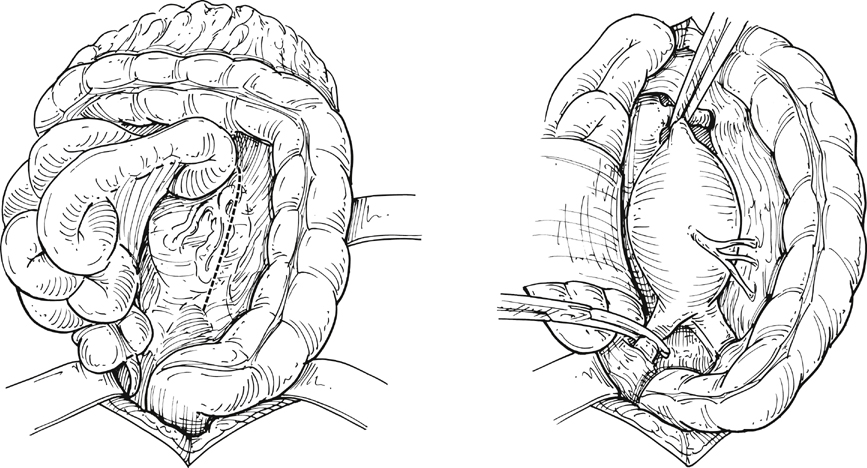
Following the abdominal exploration, the greater omentum and transverse colon are lifted superiorly and the small bowel is mobilized to the right side of the abdomen to expose the retroperitoneum. The retroperitoneum is then incised starting at the aortic bifurcation and moving superiorly, to the right of the inferior mesenteric vein, toward the ligament of Treitz to provide adequate exposure of the aortic neck region. The left renal vein can be visualized at this stage, and its absence should alert the surgeon to the possibility of a retroaortic position. The torrential hemorrhage from inadvertent injury of the anomalous left renal vein can be avoided by careful retraction and more careful placement of the aortic clamp (Figure 1).
Open Transperitoneal Surgical Treatment of Nonruptured Infrarenal Aortic Aneurysms
Technique
Exposure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine