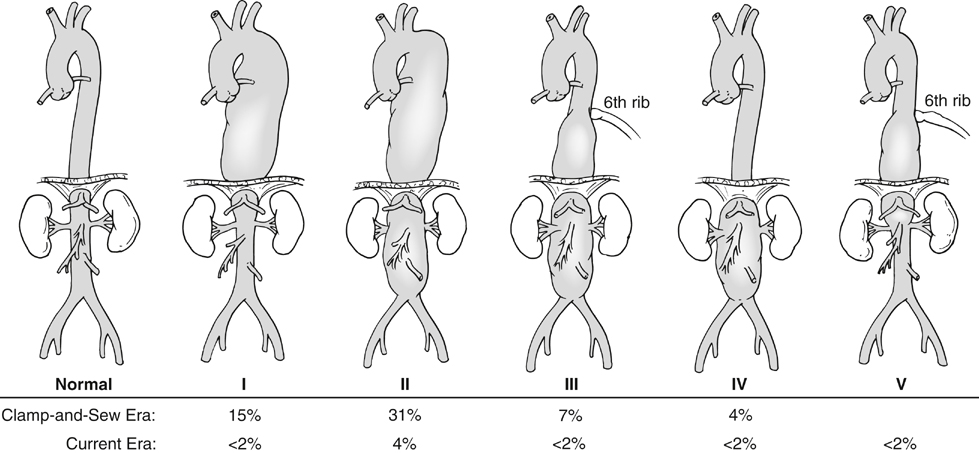
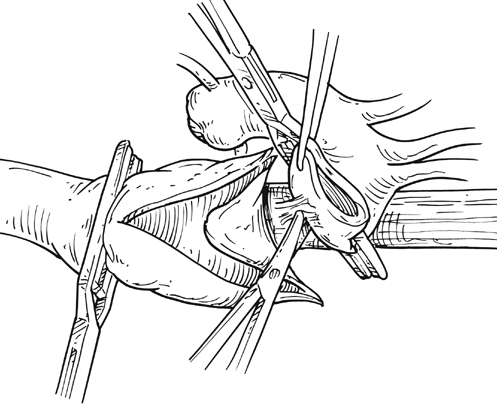
Kristofer M. Charlton-Ouw, Anthony L. Estrera and Hazim J. Safi The classification of thoracoabdominal aortic aneurysms is based on the extent of the involved aorta (Figure 1). Extent I is from the left subclavian to above the most proximal renal artery. Extent II is from the left subclavian artery to below the renal arteries. Extent III is from the sixth intercostal space to below the renal arteries. Extent IV is from T12 to below the renal arteries. Extent V, which was introduced in the last 2 decades, is from T6 to just above the renal arteries. The importance of the classification scheme is that it correlates with the incidence of neurologic deficits and mortality, especially when using the clamp-and-sew technique. Anticoagulation is started, and the inflow cannula is placed for distal aortic perfusion in the left inferior pulmonary vein. The outflow cannula is placed on the left common femoral artery using an end-to-side Dacron graft. The side-arm femoral cannulation technique prevents muscle ischemia and injury of the kidneys by allowing perfusion to the extremity. Distal aortic perfusion is initiated by withdrawing blood from the pulmonary vein and, using a roller pump, directing flow into the femoral cannula. When the aorta is clamped proximal to the aneurysm, flow is maintained to the viscera, renals, and lower extremities. A distal clamp is applied, and the aortic segment between the two clamps is opened (Figure 2). The aorta is separated from the esophagus, and the proximal anastomosis is sewn end to end with 3–0 polypropylene suture. The distal clamp is sequentially moved down the aorta to limit branch vessel ischemia.
Open Surgical Treatment of Thoracoabdominal Aortic Aneurysms
Classification of Thoracoabdominal Aortic Aneurysms
Open Surgical Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree