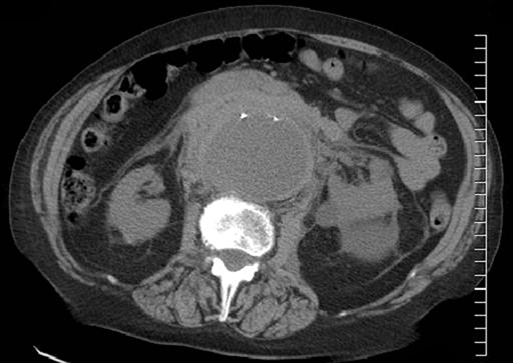
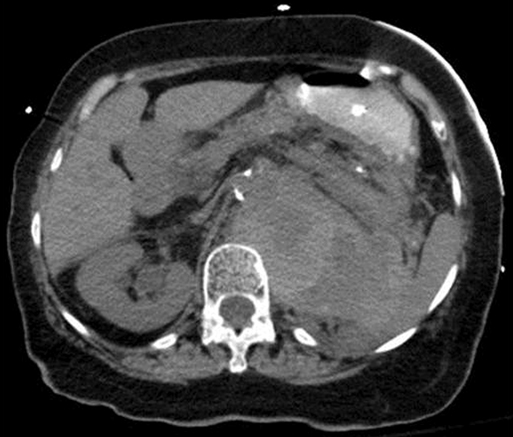
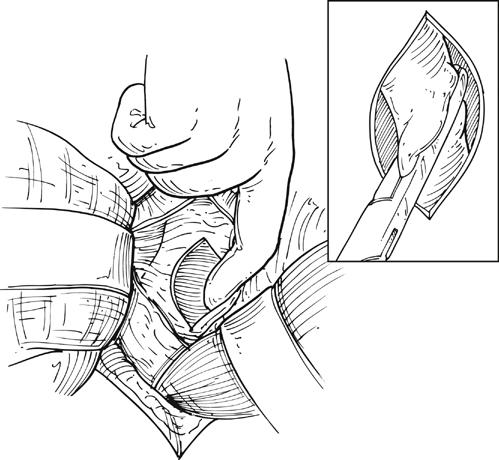
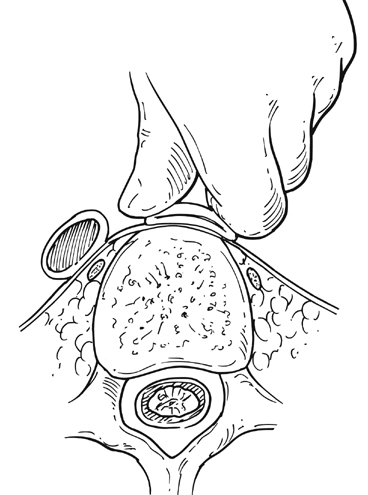
Upon arrival in the prewarmed operating room, a Foley catheter is placed, and an adequate patient warming device (Arctic Sun, Medivance, Inc, Louisville, CO) is placed. Simultaneous wide preparation of the patient, inclusive of the nipples to below the knees, is performed while intravenous and arterial access are obtained. Advanced central venous access, pulmonary artery catheters, and echocardiography probe placement should be deferred until the surgery is begun and may be done simultaneously by the anesthesia team as proximal control of the aorta is obtained. Default patient positioning is supine because more than 95% of AAAs are infrarenal and may be approached transabdominally (Figure 1). If preoperative imaging was performed and a perivisceral or thoracoabdominal component exists (Figure 2), right lateral decubitus positioning allows easier access to the entire thoracoabdominal aorta via a retroperitoneal approach. The gastrohepatic ligament is divided after palpation for a replaced or accessory left hepatic artery, which is present in 10% to 20% of patients and courses in a transverse orientation toward the left hepatic lobe. The safest practice is to directly dissect down onto the aortic pulse. A combination of sharp and blunt dissection is used to splay the fibers of the diaphragmatic crus and retroperitoneal tissues until the white sheen of the aortic wall is revealed. Gentle blunt finger dissection is used directly on the aorta laterally and medially to develop enough space to insert a deep aortic clamp into place (Figure 3). If at any point during this dissection the aortic pulse is lost, immediate aortic control may be obtained by direct digital compression of the aorta posteriorly onto the vertebral column (Figure 4).
Open Surgical Treatment of Ruptured Infrarenal Aortic Aneurysms
Procedure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
