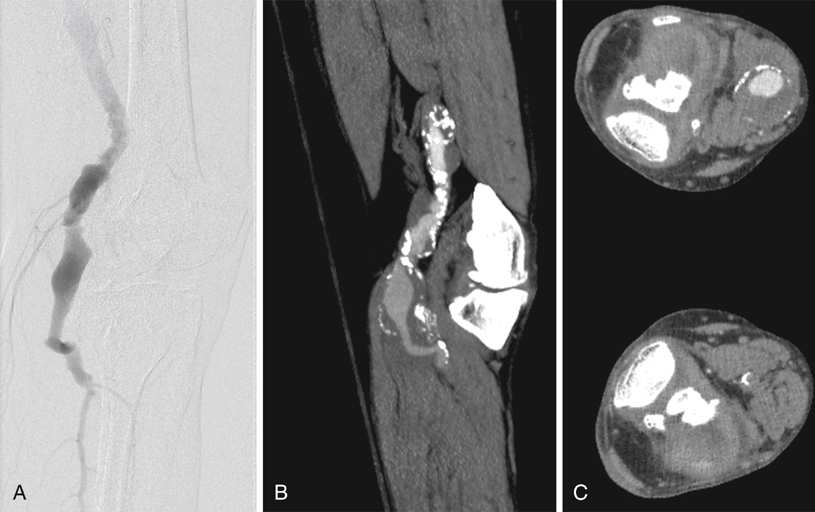
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) offer noninvasive ways to obtain information regarding the extent of the aneurysm and the status of runoff vessels. Images obtained through these modalities can be used to determine the best surgical approach for repair. CTA is especially useful if consideration is to be given to an endovascular repair. Conventional angiography provides only information about the internal diameter of a vessel and thus can underestimate the diameter of a PAA containing thrombus (Figure 1). The principal role of conventional angiography lies in its use for evaluating runoff vessels, particularly in the acute setting, and in initiating and follow-up imaging of thrombolysis. An analysis of the Swedvasc registry compared 1-year results between medial and posterior approaches. The medial approach was used just over 10 times more often than the posterior approach. Neither 30-day (93.0% vs. 93.9%) nor 1-year (87.0% vs. 90.3%) patency rates were statistically different between the posterior and the medial approaches, respectively (Table 1). The authors also found that these two approaches also had similarly low (3.7% vs. 2.6%) late amputation rates resulting from either of the two procedures. Some authors have offered dissenting opinions, with single-center data demonstrating superior patency and limb salvage with the posterior approach. TABLE 1 Patency Rates Comparing Medial and Posterior Approaches to Popliteal Artery Aneurysm Repair
Open Surgical Treatment of Popliteal Artery Aneurysms
Diagnosis and Imaging
Surgical Management
Repair
1-Year Patency (%)
Long-Term Patency
Author
Approach
Total
Sympt
Asympt
Vein
PTFE
30-Day
Patency (%)
P
PA
S
Years
P (%)
PA (%)
S (%)
Galland
Medial
28
12
16
20
8
100
ns
ns
ns
5
78∗
Kropman
Medial
33
20
13
24
9
100
93
96
96
4
69
78
90
Posterior
33
21
12
24
9
94
79
80
100
4
66
80
90
Ravn
Medial
390†
ns
ns
312
68
94
90∗
7
83∗,‡
Posterior
57†
ns
ns
38
19
93
87∗
7
82∗,‡
Zaraca
Medial
11†
ns
ns
ns
ns
ns
ns
ns
ns
6
100
100
91
Posterior
38†
ns
ns
ns
ns
ns
ns
ns
ns
6
94
97
97
Beseth
Posterior
30
28
2
0
30
100
92
96
96
ns
ns
ns
ns ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Open Surgical Treatment of Popliteal Artery Aneurysms
