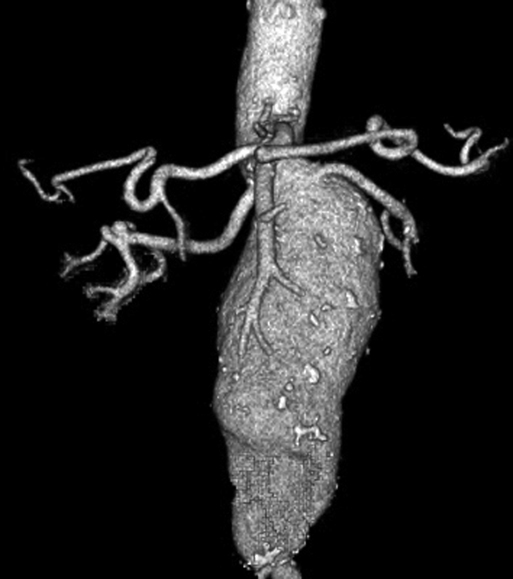
In general, indications for surgical treatment of pararenal AAAs are the same as for infrarenal AAAs, including a diameter of 5 cm or greater in asymptomatic patients with acceptable operative risks.
The surgical risks attending operations for pararenal AAAs are greater than for treatment of infrarenal AAAs. Three obvious factors contribute to these risks. First, a relatively extensive aortic dissection is required for repair of pararenal AAAs, which translates into increased operative time and greater surgical hazards. Second, intraoperative renal ischemia caused by suprarenal aortic clamping is an inescapable part of these cases. Third, cardiac strain is greater as a result of the increased afterload accompanying the more proximal aortic clamp placement in these patients.
The magnitude of treating pararenal AAAs supports a relatively universal preoperative cardiac assessment rather than the customary selective evaluation before infrarenal AAA repair. Noninvasive pharmacologic or exercise cardiac testing is recommended in all patients with pararenal AAAs, and cardiac catheterization is pursued if myocardial ischemia is suspected. Perioperative monitoring with peripheral and pulmonary arterial lines is essential to guide afterload reduction and fluid support. Transesophageal echocardiography is often used to assess cardiac strain during aortic cross clamping.
Pararenal AAA exposure is more critical and controversial than exposure for infrarenal AAA repair. Four basic approaches to pararenal AAAs are appropriate: transabdominal, retroperitoneal, thoracoretroperitoneal, and thoracoabdominal. A direct abdominal approach with a supraumbilical transverse incision, which is favored over a midline incision, is used in treating most pararenal AAAs. Direct exposure of the pararenal aorta at the base of the mesentery and mesocolon is usually pursued, although exposure following medial visceral rotation is often undertaken in the case of unusually large pararenal AAAs. Although medial visceral rotation yields excellent exposure of the aneurysm neck and visceral vessels, pancreatitis is a potential complication.
Regardless of which approach is taken, the pancreas is elevated superiorly and the crura of the diaphragm on both sides of the aorta are transected perpendicular to their fibers, using electrocautery. This provides a relatively unencumbered space about the pararenal aorta. Fixed retractors facilitate this exposure, but they must be positioned with care to prevent organ trauma.
A retroperitoneal exposure through a flank or thoracic incision is preferred in certain settings. This approach is associated with significantly less postoperative fluid requirement and a shorter hospital stay, but no difference in morbidity or mortality has been documented. Unfortunately, if a right renal artery or iliac artery reconstruction is required, it may be technically more difficult using this approach. In such circumstances a second abdominal incision is usually required. The various approaches all provide reasonable exposure of pararenal AAAs in experienced hands, and the choice is often dictated by the surgeon’s preference or by other factors, such as the location of previous abdominal incisions.
The safest site for placing the proximal aortic clamp at the supraceliac, supramesenteric, or suprarenal level is controversial. The patient’s anatomy and disease are often the main determinants of clamp placement. Although no difference in patients’ morbidity or mortality was found in one series among any of these three clamp sites, especially in regard to postoperative renal insufficiency or coagulopathy, others believe that supraceliac control decreases mortality and postoperative kidney failure.
Supraceliac aortic clamping is usually easier in terms of dissection, and the aorta at this level is usually less atherosclerotic. Noncircumferential dissections of the proximal supraceliac aorta can decrease the risk of dislodging atherosclerotic debris. This site provides positioning of the clamp away from the renal artery orifices and anastomosis area. However, many have reported an increased incidence of coagulopathy and cardiac morbidity following supraceliac clamp placement.
Significant morbidity and mortality also have been observed when clamping the proximal aorta between the renal and superior mesenteric arteries or between the superior mesenteric and celiac arteries. A clamp should be placed below the superior mesenteric artery if at least a 2 to 3 cm distance exists between the former vessel and the renal arteries and only if that segment of aorta is free of intraluminal thrombus or extensive atherosclerosis. The initial position of the proximal aortic clamp must be chosen carefully because unclamping and repositioning of the clamp to a more proximal site has been associated with atheroembolization, visceral infarction, and death. Once the pararenal aorta has been exposed, isolating and occluding the renal and mesenteric arteries with microvascular clamps before proximal aortic clamping should decrease the risk of atheroembolic complications involving the kidneys and intestines, although this benefit has not been critically studied.
A dominant cause of morbidity and a substantial cause of mortality following pararenal AAA repair is kidney failure. Minimizing kidney ischemia during performance of the proximal aortic anastomosis to less than 30 minutes can decrease the incidence of postoperative renal insufficiency. The proximal aortic anastomosis is often completed in a more expeditious manner if the infrarenal aorta is transected and transposed to above the left renal vein, where the anastomosis to the graft can be completed in an easier manner. Following completion of this anastomosis, the graft can be repositioned beneath the left renal vein. Replacing the clamp to the graft below the renal arteries and restoring flow to the kidneys after the proximal aortic anastomosis us completed will decrease renal ischemia, but caution must be taken to flush any aortic debris loosened by the proximal clamp, before restoring renal blood flow. If debris is not flushed, loose material can embolize to the kidneys.
Another technique to minimize renal ischemic injury is to lower the kidney’s metabolism by infusing chilled (4°C) solution of Ringer’s lactate to which is added 1000 U heparin, 12.5 g mannitol, and 44.6 mEq of sodium bicarbonate to make 1 L. Approximately 150 mL of this solution is infused through each renal artery orifice, and administration is repeated if the occlusion time exceeds 30 minutes. This practice may be most efficacious for patients with preoperative renal insufficiency. Ice-slurry packing of the kidneys can also lower the core temperature of the kidneys and can be renoprotective.
Blood scavenging with reinfusion using a cell-saver intraoperatively is useful to decrease transfusion requirements. Limiting the dose of heparin can reduce the risk of coagulopathy, and some authors use no heparin with proximal clamp placement. However, because of a concern for thrombus formation within the renal circulation, most patients should be anticoagulated with intravenous heparin before vascular occlusion.
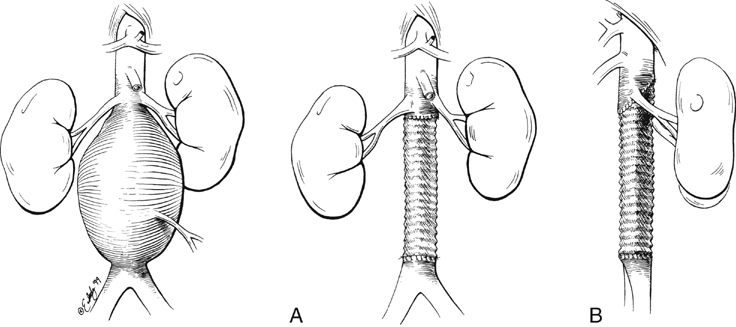
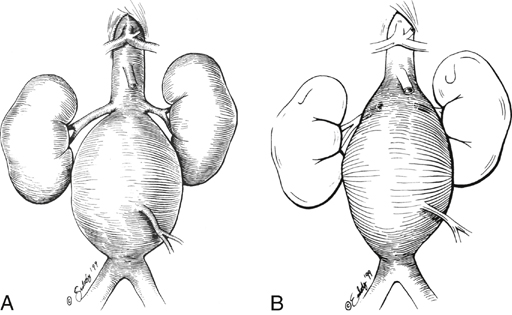
A variety of grafting techniques are employed to treat pararenal AAAs. In most patients with juxtarenal aneurysms the proximal graft-to-aorta anastomosis occurs at the level of the renal artery orifices (Figure 3A). In some of these patients, the graft is carried posteriorly above the renal arteries (Figure 3B). In other instances, anastomoses incorporate the proximal aorta and one renal artery with reimplantation of the other renal artery (Figure 4). In the case of most suprarenal aneurysms, the graft anastomosis is above the renal vessels, with bilateral reimplantation of the renal arteries (Figure 5A); an alternative is a nonanatomic renal revascularization, with grafts to the kidney originating from the supraceliac aorta or in some instances as hepatorenal or splenorenal bypasses (Figure 5B).
Only gold members can continue reading.
Log In or
Register to continue
Related
![]()