Acute proximal aortic dissection (DeBakey types I and II, Stanford type A) has traditionally been considered an absolute indication for emergency surgical repair because purely medical treatment is associated with a high risk of fatal complications. Some groups advocate delaying the repair of acute proximal aortic dissection in patients with specific risk factors, including advanced age, severely compromised physiologic reserve, previous cardiac surgery, catastrophic malperfusion (e.g., bowel infarction, stroke), delayed presentation (>48 hours), and needing transport to specialized centers.
In surgical repairs, a median sternotomy is performed to access the heart and proximal aorta. Most commonly, cardiopulmonary bypass is established through an inflow cannula in the right axillary artery, and a two-stage venous return cannula inserted through the right atrium into the inferior vena cava. Axillary artery cannulation can be performed either directly or with an 8-mm graft conduit. The advantages of axillary artery cannulation are that it simplifies the delivery of antegrade cerebral perfusion, avoids malperfusion, and has been shown to improve survival and decrease stroke risk. Femoral artery cannulation was often used in the past but is currently less favored because of the risk of retrograde atheroembolization and malperfusion. Additional options for arterial cannulation include direct ascending aortic cannulation, innominate artery cannulation, and advancing the cannula into the ascending aorta by way of the left ventricular apex.
Limited dissections of the proximal aorta can be repaired by clamping the distal aorta and using cardiopulmonary bypass. However, most surgeons prefer the open distal technique to fully evaluate the extent of arch involvement, which requires a period of hypothermic circulatory arrest. In addition, placing a distal aortic cross clamp can limit aortic resection or fracture the delicate tissue that separates the true and false lumens, producing a new reentry point beyond the distal anastomosis.
Three methods of cerebral protection are used during proximal aortic dissection repair: hypothermic circulatory arrest, retrograde cerebral perfusion, and antegrade cerebral perfusion. Hypothermia decreases metabolic activity, allowing safe circulatory arrest. If used without cerebral perfusion, hypothermic circulatory arrest can be maintained only for a limited time without increasing the patient’s risk of stroke and death.
Retrograde cerebral perfusion delivers cold oxygenated blood from the pump through a cannula in the superior vena cava. Although this technique was widely used in the past and is still used by some groups, many believe its efficacy is limited. Antegrade perfusion allows direct perfusion of the brachiocephalic arteries through flexible balloon catheters or through an axillary artery cannula.
If antegrade cerebral perfusion can be established, the distal anastomosis and arch repair (if needed) can be conducted under moderate hypothermia (23°C–25°C). Historically, circulatory arrest was initiated with profound hypothermia (<18°C), which necessitated prolonged cardiopulmonary bypass and caused coagulopathy. Recent experience has shown that moderate hypothermia with cerebral perfusion provides good cerebral protection with shorter bypass times and less coagulopathy. Once the target temperatures are reached, bypass flows are kept at 1.0 to 1.5 L/min. The innominate artery is occluded with a snare, which initiates antegrade right cerebral perfusion and circulatory arrest to the body. Once the aorta is opened, left carotid perfusion with a separate balloon catheter can be selectively used according to the anticipated duration of circulatory arrest and readings from near-infrared spectroscopy cerebral monitoring.
After the ascending aorta is opened, a decision can be made regarding the extent of distal aortic repair. Options include ascending aorta repair only, beveled hemiarch repair, total arch replacement, and elephant trunk repair. Advocates of total arch replacement state that it is generally indicated if the primary intimal tear is located in the arch or if the arch is aneurysmal. The goal is to resect the primary aortic tear site to depressurize the distal false lumen and decrease the risk of a descending thoracic aortic aneurysm developing in the residual dissected aorta. The use of selective cerebral perfusion has extended the safe period of circulatory arrest, allowing optimal repair of the aortic arch without increasing operative risk. Those who support limited resection of the arch argue that total arch replacement increases mortality risk. In addition, they cite the lack of evidence for a survival benefit or a lower incidence of late distal aneurysms when the entire arch is replaced.
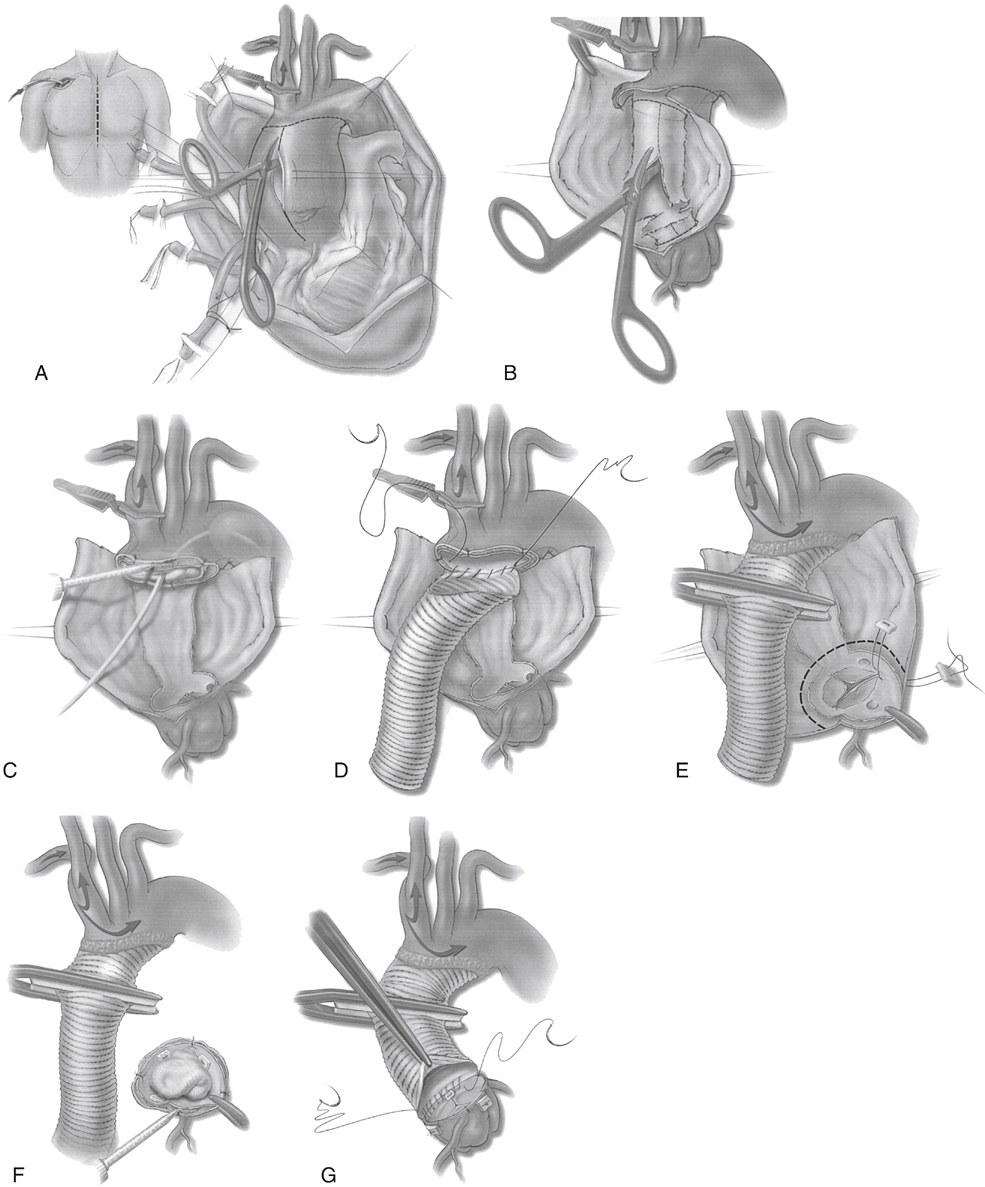
If the entire arch is not involved, a less extensive beveled hemiarch repair is performed ( Figure 2 ). First, the dissecting membrane that separates the true and false lumens is excised to the distal aortic cuff (Figure 2 B). The inner and outer walls are tacked together, and a surgical adhesive is sometimes used to obliterate the false lumen. A 30-mL Foley catheter balloon can be inflated in the distal aortic arch to prevent surgical adhesive from migrating distally in the false lumen (Figure 2 C). A polyester tube graft is sutured to the distal aortic cuff (Figure 2 D). Generally, 26- to 30-mm diameter tube grafts are used. If the aortic wall is particularly friable, the suture line may be buttressed with a second suture line or interrupted pledgets. The snare around the innominate artery is released, the graft is deaired and clamped, and full cardiopulmonary bypass is resumed. Warming is started, and the proximal portion of the repair is begun.
FIGURE 2 Technique for surgical repair of acute DeBakey type I aortic dissection. A, The ascending aorta is opened during hypothermic circulatory arrest supplemented with antegrade cerebral perfusion. B, To expose the true lumen, the dissecting membrane is removed. C, The false lumen is obliterated by using surgical adhesive. A balloon catheter and moist sponge are placed in the true lumen to prevent unwanted spread of the adhesive. D, The open distal anastomosis technique prevents clamp injury of the arch tissue and permits visual inspection for tears. E, The distal anastomosis is secured with additional adhesive, and the aortic valve is resuspended to restore valvular competence. F, Proximally, the aorta is commonly transected at the sinotubular junction. After placing a protective moist sponge in the true lumen of the aortic valve, adhesive is used to obliterate the false lumen. G, Once the adhesive has set, the proximal anastomosis is performed at the sinotubular junction and incorporates the distal margin of the commissures. (Reproduced from Creager MA, Dzau VS, Loscalzo J (eds): Vascular Medicine , Philadelphia, 2006, WB Saunders, with permission. Figure 35.3. Copyright Saunders/Elsevier, 2006.)
Only gold members can continue reading.
Log In or
Register to continue
Related 
![]()



