Open Surgical Revascularization for Femoropopliteal and Infrapopliteal Arterial Occlusive Disease
David K.W. Chew
Michael Belkin
The current practice of vascular surgery encompasses a wide spectrum of procedures, ranging from percutaneous endovascular interventions to standard open vascular reconstructions. Among this diversity of technical skills required of the contemporary vascular surgeon, infrainguinal arterial bypass surgery is still generally considered the signature operation distinguishing the vascular surgeon from other specialists who treat peripheral vascular disease. One reason why infrainguinal arterial bypass surgery has earned this honorable distinction is because the results of this procedure are highly dependent on the technical skill of the surgeon, with the outcome being either successful limb salvage or major amputation of the limb. Therefore, all vascular surgeons should master infrainguinal arterial bypass surgery and endeavor to perform this operation well. This chapter focuses on both standard and advanced techniques in infrainguinal bypass surgery.
Indications and Contraindications
Patients with chronic arterial occlusive disease of the femoropopliteal and infrapopliteal vessels present with varying degrees of ischemia of the lower limb, clinically manifesting as calf claudication, ischemic rest pain, or loss of tissue in the foot. The classic indications for surgical revascularization are disabling claudication and limb salvage in patients with critical limb ischemia (defined as ischemic rest pain, ulceration, and gangrene). Less common indications for infrainguinal arterial bypass surgery include trauma (e.g., popliteal artery occlusion from posterior knee dislocation), popliteal artery entrapment syndrome, and femoropopliteal arterial aneurysm with thrombo-embolism.
Infrainguinal arterial reconstruction should not be performed for nondisabling claudication, in severely debilitated patients with prohibitive comorbidities, or in patients who are bedridden or who have severe joint contractures. In patients who do not ambulate but require the use of their limb for balance in a wheelchair and bed transfers (e.g., paraplegic patients due to spinal cord injury), infrainguinal bypass surgery for arterial occlusive disease may be considered for limb salvage.
Pre-operative Assessment
The clinical diagnosis of significant lower-limb ischemia should be confirmed by noninvasive arterial testing using segmental pressures, ankle-brachial indices (ABI), and pulse-volume recordings (PVR). These studies can often identify the level of disease in the lower limb (e.g., iliofemoral, femoropopliteal, and tibial) and indicate the severity of the ischemia. Furthermore, this pre-operative baseline study will aid in the follow up of patients after surgical revascularization has been performed.
When the indications for surgery have been met, the exact procedure required depends on the pathologic arterial anatomy. Classically, this has been defined by a diagnostic aortogram with lower-extremity runoff using intra-arterial contrast and digital subtraction imaging. Although the information obtained from such a study is usually excellent, the disadvantages of this procedure include its invasive nature and the risk of contrast-induced nephrotoxicity and allergic reaction. In patients with diabetes mellitus (DM) and chronic renal insufficiency (CRI), the risk of contrast-induced renal failure is significant. Preprocedural intravenous hydration, oral acetyl-cysteine, and the use of newer generation isoosmolar, non-ionic contrast medium (e.g., VisipaqueTM) may minimize this risk but do not eradicate it. Carbon dioxide and gadolinium have been used as alternative contrast agents, but the image resolution of the arterial anatomy is suboptimal and gaseous bubbling may induce artifacts that mimic stenotic lesions.
Recently, magnetic resonance angiography (MRA) using time-of-flight sequences and gadolinium enhancement has emerged as an alternative study of choice for preoperative planning. This noninvasive study offers good imaging of the arterial anatomy and does not impose any risks of renal toxicity or radiation exposure. With current technology, the quality of the images is usually limited only by the experience of the imaging technician and has improved with the adoption of standardized protocols and increasing experience with these studies. Other authors have relied solely on duplex ultrasonography of the tibial arteries for preoperative planning. This is a time-consuming and technically demanding diagnostic procedure, which is not practical in most high-volume vascular laboratories.
Once the anatomy has been clearly defined and the magnitude of the planned vascular reconstruction determined, it is important to evaluate the patient’s fitness for surgery. Myocardial infarction (MI) is the major cause of peri-operative morbidity and mortality. The incidence of coronary artery disease (CAD) in patients presenting with significant lower-limb ischemia is as high as
50%. Objective cardiac risk stratification often necessitates some form of provocative cardiac stress testing, because these patients have poor exercise tolerance (e.g., persantine-sestamibi myocardial scan or dobutamine stress echocardiogram). Any suggestion of significant myocardium that may be at potential risk for infarction should be selectively evaluated with coronary angiography. In general, coronary arterial disease is treated on its own merits, and this is performed before infrainguinal arterial bypass surgery. Routine medical measures include control of blood pressure and heart rate with beta-blockade, antiplatelet therapy with aspirin, lipid-lowering with statin therapy and normalization of blood glucose levels in diabetics. Patients are advised to stop smoking at least 2 weeks before surgery.
50%. Objective cardiac risk stratification often necessitates some form of provocative cardiac stress testing, because these patients have poor exercise tolerance (e.g., persantine-sestamibi myocardial scan or dobutamine stress echocardiogram). Any suggestion of significant myocardium that may be at potential risk for infarction should be selectively evaluated with coronary angiography. In general, coronary arterial disease is treated on its own merits, and this is performed before infrainguinal arterial bypass surgery. Routine medical measures include control of blood pressure and heart rate with beta-blockade, antiplatelet therapy with aspirin, lipid-lowering with statin therapy and normalization of blood glucose levels in diabetics. Patients are advised to stop smoking at least 2 weeks before surgery.
Pre-operative vein mapping with duplex ultrasonography is useful for guiding the selection of alternative autogenous conduits in patients in whom both greater saphenous veins (GSV) are absent or suspected of being diseased (e.g., thrombosis, sclerosis, or varicosities). Ideally, this study should be performed with the superficial veins in a distended state by placing a tourniquet in the proximal arm or leg and with the extremity in a dependent position. In the absence of usable GSV, the cephalic vein (CV), basilic vein (BV), and lesser saphenous vein (LSV) should be studied.
Principles of Open, Infrainguinal Revascularization
The first prerequisite for the successful performance of infrainguinal arterial bypass grafting is to ensure that there is adequate inflow into the artery from which the bypass graft is originating. If the inflow artery is inadequate, preliminary procedures such as iliac angioplasty/stent or a surgical inflow procedure will need to be performed prior to construction of the infrainguinal arterial bypass graft. The most acceptable distal inflow vessel is chosen for origination of the bypass graft.
Secondly, the target vessel chosen as the outflow vessel should be the least diseased vessel that is the dominant blood supply to the foot. In the presence of tissue necrosis, restoration of pulsatile flow to the foot is preferred to maximize the chances for wound healing. We have not found the site of the distal anastomosis per se (i.e., popliteal vs. tibial/pedal) to influence the long-term patency of the bypass graft. Bypass grafts performed to distal tibial/pedal target vessels may not relieve calf claudication if this is the primary indication for surgery.
Thirdly, the preferred conduit for arterial reconstruction is the GSV, even for reconstructions to the above-knee popliteal artery. In elderly patients with significant medical comorbidities where long-term graft patency may not be as relevant, it is reasonable to use a prosthetic graft, e.g., polytetrafluoroethylene (PTFE) or Dacron, for bypass to the popliteal artery. In the absence of ipsilateral GSV, the contralateral GSV is the next conduit of choice unless the contralateral lower limb is also severely ischemic and in need of bypass surgery. When both GSVs are unavailable, alternative vein conduits (CV, BV, and LSV), including autogenous composite vein (ACV) grafts, are used. Due to the poor performance of prosthetic grafts for infrageniculate arterial reconstruction, all autogenous options are exhausted before such grafts are considered for infrapopliteal bypasses.
The GSV is harvested starting below the groin crease and proceeding distally. There are several configurations in which the GSV can be used:
In situ bypass technique
Reversed
Nonreversed, transposed
Equivalent long-term results have been reported with all three configurations. The in situ technique emphasizes leaving the GSV in its vein bed with ligation of the tributaries and lysis of valves. Theoretical advantages of this approach include minimal “disruption” of the nutrient supply to the vein wall and optimization of the sizematch between the vein graft and the native vessels. Our preference, however, is to use the GSV in the nonreversed, transposed configuration, as this achieves the above advantage of size optimization between the vein graft and the native vessels; in addition, it offers the flexibility of being able to move the vein graft to more distal inflow sites. In our practice, vein grafts are used in a reversed orientation only if the caliber of the graft is uniform throughout. Ideally, vein segments should have a minimum diameter of 3.5 mm, distend easily with irrigation, and have no evidence of significant wall thickening and sclerosis/thrombosis. Vein segments that do not meet these criteria are excised, and composite vein grafting is performed. The vein graft is preferably placed in a subcutaneous location to facilitate postoperative graft surveillance using duplex ultrasound and graft revision if necessary. Finally, a completion study (contrast angiogram and/or duplex ultrasonography) should be performed to assess the technical adequacy of the bypass procedure.
Operative Technique
Patient Preparation
Infrainguinal arterial reconstruction can be performed under regional anesthesia (e.g., continuous epidural) or general anesthesia, depending on the medical condition of the patient and whether there is a need to harvest arm veins. The patient is placed in a supine position with both arms extended. A Foley catheter is passed into the bladder. Standard betadine preparation and draping of the extremities are performed. Prophylactic antibiotics are given intravenously. A radial arterial line is usually placed for hemodynamic monitoring and drawing of blood samples for activated clotting time (ACT) measurements.
Exposure of the Arteries
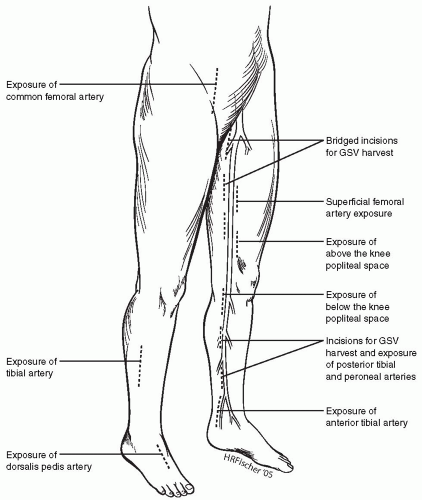
The skin incisions for exposing the lower-extremity arteries and the greater saphenous vein are shown in Figure 51-1.
Common Femoral Artery (CFA)
A short longitudinal or oblique incision is made directly over the femoral pulse from the inguinal ligament caudal. When possible, it is better to avoid incising across the groin crease, as this is a common site for wound breakdown due to hip flexion. The dissection is centered directly over the CFA to avoid the creation of skin flaps. To prevent lymph leaks and seroma formation, the lymph nodes are dissected laterally and not directly transected. All lymphatic vessels emanating from the nodes are ligated before division. Self-retaining Weitlaner retractors are useful for exposing the wound, but prolonged traction on the skin edges may lead to pressure-induced necrosis. The CFA, superficial femoral artery (SFA), and profunda femoris artery (PFA) are dissected out and isolated with vessel loops. Care must be taken to avoid injury to the large vein that crosses over the proximal PFA just beyond the CFA bifurcation. This needs to be ligated with 3-O silk ties and divided for more generous exposure of the PFA.
When the CFA is severely calcified and unclampable, division of the inguinal ligament and exposure of the external iliac artery in the retroperitoneum should be performed. At the conclusion of the procedure, the inguinal ligament should be repaired with interrupted, horizontal mattress sutures
(e.g., using O Vicryl) to prevent postoperative inguinal herniation. A severely diseased CFA may require thrombo-endarterectomy with prosthetic patch angioplasty (e.g., using bovine pericardium) before it is suitable for origination of the infrainguinal bypass graft. Patency of the PFA is restored by removing all obstructive plaque in this vessel, ensuring that the distal intima is adherent or incorporating the patch closure of the CFA into the PFA (profundaplasty). Restoration of a normal PFA is critical and may ensure limb viability even when the bypass graft occludes in the future.
(e.g., using O Vicryl) to prevent postoperative inguinal herniation. A severely diseased CFA may require thrombo-endarterectomy with prosthetic patch angioplasty (e.g., using bovine pericardium) before it is suitable for origination of the infrainguinal bypass graft. Patency of the PFA is restored by removing all obstructive plaque in this vessel, ensuring that the distal intima is adherent or incorporating the patch closure of the CFA into the PFA (profundaplasty). Restoration of a normal PFA is critical and may ensure limb viability even when the bypass graft occludes in the future.
Superficial Femoral Artery (SFA)
When the SFA is selected as the inflow vessel, a longitudinal incision is made in the anteromedial aspect of the thigh overlying the sartorius muscle. For exposure of the proximal SFA, the sartorius is reflected laterally; and for the distal SFA, it is reflected posteriorly. Beware of the adjacent superficial femoral vein and saphenous nerve that run with it. Injury to this nerve causes neuralgic pain and numbness along the anteromedial aspect of the thigh and leg.
Above-knee Popliteal Artery (AK Pop)
The knee is flexed by placing a roll under the calf. A longitudinal incision is made in the medial aspect of the distal thigh below the muscle belly of the vastus medialis. The sartorius muscle is reflected posteriorly, and the above-knee popliteal fat pad is entered. Dissection is then performed close to the posterior aspect of the femur, and the adductor magnus tendon will be seen. The SFA enters the popliteal space after crossing the adductor hiatus and is then called the popliteal artery. Beware of the saphenous nerve, which may be injured as self-retaining retractors are placed to keep the popliteal space open.
Below-knee Popliteal Artery (BK Pop), Tibioperoneal Trunk (TP Trunk)
The knee is flexed by placing a roll under the distal thigh. A longitudinal incision is made in the medial aspect of the upper calf overlying the course of the GSV. The GSV is carefully dissected out and mobilized. The incision is then deepened through the fascia with the electrocautery. The medial head of the gastrocnemius muscle is reflected posteriorly and the below-knee popliteal space entered. Exposure is best maintained by using angled Weitlaner (“cerebellar”) or Adson-Beckman retractors. The popliteal vein will be visualized, and in a more posterior plane, the tibial nerve will also be visualized. The popliteal artery can be seen closely adherent to the paired popliteal veins on either side. Careful sharp dissection using Metzenbaum scissors while maintaining gentle traction on the artery with a vessel loop will facilitate its mobilization.
To expose the TP trunk, follow the popliteal artery distally and divide the overlying soleus muscle insertion onto the tibia with the electrocautery. The anterior tibial veins need to be ligated with 3-O silk ties and divided before the distal popliteal vein can be rotated posteriorly to expose the tibioperoneal trunk. The origin of the anterior tibial artery, which is located at the upper end of the soleus insertion, is then isolated with a vessel loop.
Posterior Tibial (PT) and Peroneal (Per) Arteries
A longitudinal incision is made in the medial aspect of the leg overlying the GSV. The GSV is carefully dissected out and mobilized. The incision is then deepened through the fascia with the electrocautery. As the muscular insertion of the soleus muscle into the posterior aspect of the tibia is taken down, the deep posterior compartment of the calf will be entered. The posterior tibial artery lies superficially in this plane (above the flexor digitorum longus) and is accompanied by the paired venae comitantes. Dissection of the artery is best performed with a pair of Metzenbaum scissors.
The peroneal artery lies deeper in the same compartment, close to the fibula bone. Dissection is performed deep to the flexor hallucis longus muscle in the upper calf. This artery is similarly entwined by its paired venae comitantes. Excision of these veins will facilitate exposure of the artery.
With the use of tourniquet control of the vessels, only the anterior and lateral aspects of the tibial vessels need to be exposed. There is no need to perform circumferential mobilization of these small arteries. The plane of dissection for exposure of the tibial vessels is illustrated in Figure 51-2.
With the use of tourniquet control of the vessels, only the anterior and lateral aspects of the tibial vessels need to be exposed. There is no need to perform circumferential mobilization of these small arteries. The plane of dissection for exposure of the tibial vessels is illustrated in Figure 51-2.
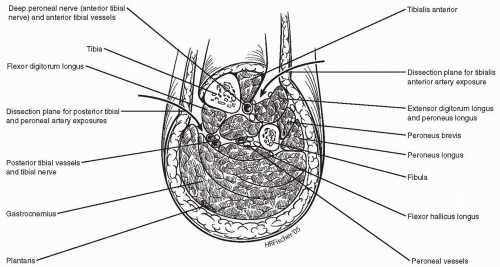 Figure 51-2. A cross-sectional illustration of the mid calf is shown with the dissection planes for the posterior tibial/peroneal and anterior tibial arteries identified. |
In some instances, the most distal peroneal artery may be approached laterally via an incision placed over the distal fibula. A segment of the fibula may then be excised in order to expose the peroneal artery.
Anterior Tibial (AT) Artery
A longitudinal incision is placed in the anterolateral compartment of the leg, just lateral to the tibialis anterior muscle belly. The intermuscular plane between this muscle and the extensor digitorum longus is developed. The anterior tibial vessels with the deep peroneal nerve will be found in this plane, on top of the interosseous membrane (Fig. 51-2). Due to the bulky muscles proximally, it is easier to expose and work on the AT artery lower down in the leg as the vessel rises to a more superficial location.
Dorsalis Pedis (DP), Distal PT, and Pedal Vessels
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree