Open Surgical Revascularization for Extracranial Carotid Occlusive Disease
Ali F. AbuRahma
Stroke remains the third leading cause of death in the United States, and it is the second leading cause of death in the United States for women. It has been reported that 50% to 75% of patients suffering a stroke have surgically accessible extracranial vascular disease.
Regardless of which criteria are used to determine whether carotid endarterectomy (CEA) is warranted, a surgeon must stay within the accepted peri-operative stroke rate of <3% to 7% (depending on indication), as recommended by the Ad Hoc Committee of the Stroke Council of the American Heart Association.
Anatomic Considerations
The aortic arch gives off, from right to left, the innominate (brachiocephalic trunk), the left common carotid, and the subclavian arteries. The innominate artery passes beneath the left innominate vein before it branches into the right subclavian and the right common carotid arteries (CCA). The vertebral arteries branch off the subclavian arteries 2 or 3 cm from the arch, but many variations may occur. The left CCA may arise from the innominate and cross to a relatively normal position on the left side. The left vertebral artery may arise directly from the aortic arch, and the right vertebral artery may arise as part of a trifurcation of the brachiocephalic trunk into subclavian, common carotid, and vertebral arteries. Occasionally, the right subclavian may arise distal to the left subclavian artery and cross to the right side.
The CCAs on each side travel in the carotid sheath up to the neck before branching into internal carotid (ICA) and external carotid arteries (ECA) just below the level of the mandible. The ECA supplies the face. Important branches of the ECA that should be noted include the superior thyroid, which can actually arise from the CCA; the ascending pharyngeal, which is important in that it accompanies the superior laryngeal nerve; and the lingual and occipital arteries that have a close association with the hypoglossal nerve. No branches of the ICA occur in the neck.
The carotid sinus, a baroreceptor, is located in the crotch of the bifurcation of the ICA and ECA. It is innervated by the sinus nerve of Hering, which branches from the glossopharyngeal nerve. The carotid body is a very small structure that also lies in the crotch of the bifurcation and functions as a chemoreceptor, responding to low oxygen or high carbon dioxide levels in the blood. It is also innervated by the glossopharyngeal nerve via the sinus nerve of Hering.
The ophthalmic artery (a branch of the cavernous portion of the ICA) is clinically important because it communicates with the external carotid system, which is the basis of the peri-orbital Doppler study.
The major collateral pathway protecting the cerebral cortex is the intracranial circle of Willis. This unique circle provides the major pathway between the ICA, the ECA, and the vertebrobasilar systems.
Pathology/Pathogenesis
Atherosclerosis
Atherosclerosis accounts for approximately 90% of extracranial cerebrovascular disease, with the remaining 10% being attributed to such disease processes as fibromuscular dysplasia, traumatic or spontaneous dissection, aneurysms, and arteritis, including Takayasu arteritis.
Atherosclerotic plaques occur preferentially at areas of vessel bifurcations, and the process is similar to that seen with coronary artery disease (CAD). It often begins in the bulbous portion of the ICA on its posterior lateral wall. These plaques can enlarge in several ways; they may continue to slowly enlarge from accumulation of cholesterol and fibroblasts. Aternately, central necrosis of the plaque and rupture of the intimal lining of the vessel will lead to discharge of atheromatous debris into the lumen of the vessel as an embolus. The atherosclerotic plaque can also become a nidus for platelet deposition and thrombosis and/or further embolization to the brain. Accumulation of the arteriosclerotic plaque may result in progressive stenosis or total occlusion of the carotid artery with subsequent thrombosis of the ICA distal to the lesion. Another mechanism by which there may be sudden plaque enlargement is intraplaque hemorrhage. If the intima overlying the site of plaque hemorrhage ulcerates, the necrotic contents of the atheroma escape into the lumen and cause cerebral embolization with transient ischemic attacks (TIAs) or cerebral infarcts. The CCA bifurcation and the proximal ICA account for 50% of atherosclerotic extracranial cerebrovascular lesions. Vertebral artery lesions account for 20%, left subclavian arterial lesions account for 10% to 15%, and lesions of the innominate and right subclavian arteries account for 15%.
The most common cause of symptomatic cerebral ischemic events is an embolus. The majority are arterial in origin (carotid) with cardiac sources a distant but still significant second. If the embolism breaks up quickly
from mechanical forces or from the effect of arterial prostacycline, the symptoms will be transient, i.e., TIAs. If the embolic fragment persists, however, it can lead to focal infarction. An ICA thrombosis usually produces a column of thrombus that stops at the ophthalmic artery and remains stable if there is sufficient collateral circulation via the circle of Willis. In this instance, the thrombotic event may be entirely asymptomatic. However, if small thrombi rather than a thrombotic column form and are subsequently carried to the intracranial vessels by continuous blood flow, then the patient will experience cerebral symptoms that can vary from transient amaurosis fugax or hemispheric events to a profound fixed hemiplegia. If the collateral circulation to the circle of Willis is inadequate, the sudden loss of blood flow through a diseased ICA may induce a sudden drop in flow to the cerebral hemisphere, resulting in ischemic infarction.
from mechanical forces or from the effect of arterial prostacycline, the symptoms will be transient, i.e., TIAs. If the embolic fragment persists, however, it can lead to focal infarction. An ICA thrombosis usually produces a column of thrombus that stops at the ophthalmic artery and remains stable if there is sufficient collateral circulation via the circle of Willis. In this instance, the thrombotic event may be entirely asymptomatic. However, if small thrombi rather than a thrombotic column form and are subsequently carried to the intracranial vessels by continuous blood flow, then the patient will experience cerebral symptoms that can vary from transient amaurosis fugax or hemispheric events to a profound fixed hemiplegia. If the collateral circulation to the circle of Willis is inadequate, the sudden loss of blood flow through a diseased ICA may induce a sudden drop in flow to the cerebral hemisphere, resulting in ischemic infarction.
Clinical Syndromes and Diagnostic Considerations
The following well-defined syndromes of cerebrovascular ischemia have emerged:
Transient ischemic attacks (TIAs) are focal neurologic deficits due to cerebral ischemia that clear completely within 24 hours. However, the majority of TIAs will last only minutes to hours and in most instances the embolus arises from carotid bifurcation. This must be the site that is initially evaluated in these patients. TIAs can also occur as a result of emboli from other sites—intracranial lesions, extracranial carotid, extracranial arch vessel lesions, primary cardiac thrombus, or even paradoxical emboli. Laminar flow within the carotid vessels may repetitively send an embolus to the same area, producing nearly identical neurologic deficits.
The manifestations of carotid TIAs include transient ipsilateral blindness or visual impairment (amaurosis fugax) and contralateral sensory or motor deficit. Aphasia may be present if the dominant hemisphere is affected, and there may be a degree of altered consciousness. The patient with amaurosis fugax will describe these episodes as someone pulling a shade over one eye. Funduscopic inspection may reveal Hollenhorst plaques, bright yellow spots on the retina that represent cholesterol crystals. Homonymous hemianopsia in combination with any of the above symptoms suggests carotid TIAs.
Nonhemispheric TIAs present a dilemma to the vascular surgeon. Symptoms of dizziness, ataxia, vertigo, bilateral neurologic or visual events, or syncope may be related to lesions involving the vertebrobasilar system or to severely diminished blood flow to the brain, or diffuse global cerebral ischemia. Often such symptoms have nonvascular causes.
Crescendo TIAs are hemispheric TIAs analogous to crescendo angina. They fully resolve within minutes, but they recur with increasing frequency.
Reversible ischemic neurologic deficit (RIND) is a neurologic deficit identical to a TIA except that it takes several days for complete resolution.
Stroke in evolution causes neurologic symptoms that progress and result in permanent neurologic deficit (stroke). The symptoms may wax and wane and early on are difficult to distinguish from TIAs or RINDs.
Completed stroke is a neurologic deficit that occurs and does not have complete resolution of symptoms. This may be the result of a large embolus, a small embolus to an end vessel with surrounding vessel thrombosis, or thrombosis of the ICA.
It is important to differentiate the various etiologies that cause cerebrovascular symptoms. The workup may include echocardiograms, EEG, cerebral fluid examination, Holter monitors, and cerebral CT scanning. Arteriography should be considered if noninvasive vascular testing is equivocal. The differential diagnosis includes emboli from cardiac sources, intracerebral or intracranial hemorrhage, lacunar infarcts, and some hematologic disorders.
Pre-operative Assessment
Initial screening should always include carotid duplex scanning by an accredited vascular laboratory. Based on the findings from this study, the workup can be focused in several routes. If there is no significant disease detected by duplex, a cardiac and systemic disease workup is undertaken. If there is a severe or tight stenosis or ulcerative plaque, and the clinical scenario does not suggest another diagnosis, the patient could undergo surgery without further workup. Finally, in patients with only mild to moderate disease by duplex and hemispheric TIAs, it would be best to have other sources explored. Such patients may require carotid magnetic resonance angiography (MRA), CT scanning, arteriography, or other diagnostic considerations.
Determination of Disease Severity Using Color Duplex Ultrasound
Identification of disease in the carotid system uses both qualitative and quantitative data. Careful attention to unusual echoes on the image serves as a qualitative guide to the presence of disease at sites where careful scrutiny with a Doppler component should be performed. The changes in spectra obtained from the common, internal, and external carotid arteries provide quantitative information for the determination of the severity of disease in these locations. Multiple clinical studies have reported an overall accuracy of 80% to 97% in diagnosing carotid artery stenosis.
Duplex ultrasound is likely to remain the initial screening method of the carotid bifurcation in most centers. However, MRA may be used as a screening method in two situations:
If one plans to obtain a magnetic resonance image (MRI) of the brain to assess prior ischemic events, one can easily include screening images of the bifurcation with little additional expense
In patients whose findings are equivocal by ultrasound
CEA Based on Carotid Duplex Ultrasonography Without Angiography
In many centers, angiographic carotid evaluation is no longer routine. The risk of stroke during angiography is about 1% and the cost of angiography is $5,000 to $6,000. There is a theoretical potential to miss significant lesions in the carotid siphon or an intracranial aneurysm or tumor as the cause of symptoms. However, it is unlikely that carotid siphon disease will produce significant symptoms. Intracranial aneurysms occur in approximately 1% to 2% of patients undergoing arteriography, but most are small and unlikely to be affected by CEA. With the advances in imaging techniques, the concern for occult brain tumors has become less relevant.
Overall, CEA can safely be performed without arteriography when the following criteria are met:
The duplex scan is technically adequate.
Vascular laboratory duplex accuracy is known.
The distal ICA and CCA are free of significant disease (disease is localized to the carotid bifurcation).
Vascular anomalies, kinks, or loops are not present.
CT Scanning
Patients who present with a TIA may have actually suffered a small infarct. CT scanning or MRI can identify an unsuspected cerebral infarct and can establish a baseline status prior to CEA. This may be helpful with respect to intra-operative and postoperative management. MRI is now replacing CT scanning in some centers, because it can identify acute cerebral infarction sooner than CT scans and may show smaller infarcts that cannot be detected on CT scans.
Indications for Carotid Endarterectomy
Few surgical procedures have been scrutinized as thoroughly as CEA during the last 20 years. Several prospective randomized trials in both North America and Europe were designed to compare the safety and efficacy of CEA versus medical therapy. Collectively, the data from these prospective trials have confirmed that CEA offers significantly better protection from ipsilateral strokes than medical therapy in a substantial population of patients presenting with either symptomatic or asymptomatic carotid artery disease. The Stroke Council of the American Heart Association convened a consensus conference on the indication for CEA. Based on their recommendation, the indications for CEA can be classified as follows. Assuming symptomatic good-risk patients with a surgical morbidity and mortality (stroke and death) of <6%, proven indications (supported by prospective randomized trials) include:
One or more TIAs in the last 6 months and a carotid stenosis of ≥70%
A mild stroke with carotid stenosis ≥70%
One or more TIAs in the last 6 months and a carotid stenosis of ≥50%
Mild stroke with carotid stenosis ≥50%
Acceptable, but not proven indications include:
Ipsilateral TIA and stenosis ≥70%, combined with required coronary bypass grafting
Progressive stroke and stenosis ≥70%
Uncertain indications include:
TIA or mild stroke with <50% stenosis
Symptomatic acute carotid thrombosis
Proven inappropriate indications include:
Moderate stroke with stenosis of <50%
Single TIA with <50%, not receiving aspirin
High-risk patients with multiple TIAs or moderate stroke with stenosis of <50%, not receiving aspirin
Acute asymptomatic internal carotid dissection, receiving heparin
For asymptomatic good-risk patients treated by surgeons with surgical mortality and morbidity (stroke and death) of <3%, the indications for CEA are:
Proven: stenosis of ≥60%
Uncertain: high-risk patients or surgeons with a morbidity and mortality rate of >3%; combined carotid-coronary operation; nonstenotic ulceration lesions.
Proven inappropriate: operation with a combined stroke/morbidity rate ≥5%.
Contraindications to CEA
CEA is contraindicated if the patient’s general condition includes a serious illness that will substantially increase peri-operative risk or shorten life expectancy. CEA is contraindicated in patients who present acutely with a major stroke or in patients who had a major devastating stroke with minimal recovery or significantly altered level of consciousness. Emergency CEA in an acutely occluded carotid artery may convert an ischemic cerebral infarct to a hemorrhagic infarct, resulting in death. In any patient with a stroke (either ischemic or hemorrhagic), it is better to wait until the patient reaches optimal recovery before proceeding with elective CEA.
Operative Technique
Pre-operative Preparation
Most of our patients undergoing CEA receive general anesthesia with intra-arterial pressure monitoring, routine shunting, and preferential patching with or without intraoperative imaging. Although some surgeons prefer local or cervical block anesthesia, general anesthesia has the advantage of reducing several metabolic demands and increasing cerebral blood flow. Endotracheal intubation also provides good airway control and reduces patient and physician anxiety. Nasotracheal intubation can be used to facilitate exposure of the distal cervical segment of the ICA in patients who are known to have high carotid stenosis or in patients undergoing reoperation. Aspirin therapy is generally continued throughout the peri-operative period. The liberal use of vasopressors or nitroprussides to maintain blood pressure in the patient’s optimal physiologic range is critical.
Technique
Standard Conventional Endarterectomy
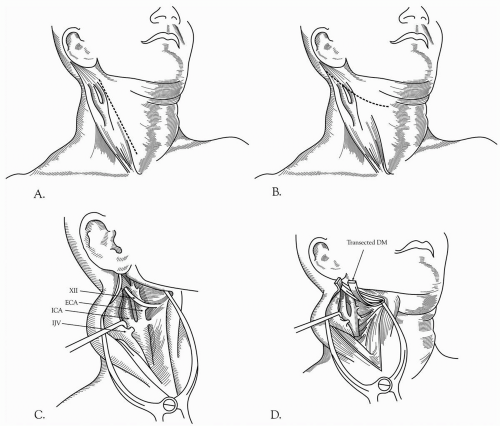
The patient is positioned supine with the head turned away from the side of the operation. The neck is moderately extended on the shoulders. Proper lighting is essential and loupe magnification routine. The cervical incision is made parallel and somewhat anterior to the sternocleidomastoid muscle and centered over the carotid bifurcation (Fig. 27-1A). This incision can be extended proximally to the sternal notch for more proximal CCA lesions, and distally to the mastoid process for higher exposure. The upper end of the incision should be angled posterior to the earlobe to avoid the parotid gland. The incision is carried down through the platysma and the stenocleidomastoid muscle laterally. Self-retaining retractors are then placed. An alternative incision placed obliquely in the skin crease over the carotid bifurcation can be used (Fig. 27-1B). After the incision is deepened through the platysma, the subplatysmal space between the sternocleidomastoid and the trachea is mobilized. This incision has the advantage of producing a more cosmetically acceptable scar than the vertical incision. However, it does have the following disadvantages:
It is more difficult to gain additional proximal or distal arterial exposure
The necessity of raising skin flaps
With either incision, subsequent steps are identical. The internal jugular vein is visualized, and the carotid sheath is opened along the anterior border of the vein. The internal jugular vein is retracted laterally, and the common facial vein is ligated (Fig. 27-1C, 27-1D, and Fig. 27-1E). Dissection is continued anterior to the CCA to avoid injury to the vagus nerve. The vagus nerve usually lies in the posterior lateral position within the carotid sheath but occasionally may spiral anteriorly, particularly in the lower end of the incision. Attention should be paid to various cranial nerves, including IX, X XI, and XIII, the marginal mandibular branch of VII, and the rare nonrecurrent laryngeal nerve that comes directly off the vagus on the way to innervate the vocal cord (Fig. 27-1E and Fig. 27-2A). This nerve can cross anterior to the carotid artery and be mistaken for a part of the ansa hypoglossi, resulting in cord paralysis. This anomaly is most often noted on the
right side of the neck (Fig. 27-2B). The vagus nerve may be closely adherent to the carotid bulb, and it becomes nearly confluent with the hypoglossal nerve near the styloid process. The CCA is generally mobilized for a sufficient length proximal to the carotid lesion. It may be necessary to inject a local anesthetic in the area of the carotid bifurcation to block the nerve to the carotid body to prevent reflex bradycardia. Dissection is continued upward to isolate the ECA. The ICA is mobilized to a point where the vessel is completely normal. The hypoglossal nerve is often surrounded by small veins that should be ligated carefully. The hypoglossal nerve may be injured by retraction; therefore, the structures that tether it in place, such as the artery and vein to the sternocleidomastoid muscle, the descending hypoglossal branch of the ansa cervicalis, and the occipital artery may require division to mobilize the nerve for distal ICA exposure. Careful attention should also be given to the superior laryngeal nerve, which is usually located medial to the ICA. The superior laryngeal nerve divides into external and internal branches that pass posterior to the superior thyroid artery, and it may be harmed while controlling either of these two vessels. The glossopharyngeal nerve crosses the ICA near the base of the skull and is best protected by maintaining dissection very close to the anterior surface of the ICA. Excessive or prolonged retraction of the upper aspect of the incision may cause temporary compression injuries laterally to the greater auricular nerve or medially to the marginal mandibular branch of the facial nerve.
right side of the neck (Fig. 27-2B). The vagus nerve may be closely adherent to the carotid bulb, and it becomes nearly confluent with the hypoglossal nerve near the styloid process. The CCA is generally mobilized for a sufficient length proximal to the carotid lesion. It may be necessary to inject a local anesthetic in the area of the carotid bifurcation to block the nerve to the carotid body to prevent reflex bradycardia. Dissection is continued upward to isolate the ECA. The ICA is mobilized to a point where the vessel is completely normal. The hypoglossal nerve is often surrounded by small veins that should be ligated carefully. The hypoglossal nerve may be injured by retraction; therefore, the structures that tether it in place, such as the artery and vein to the sternocleidomastoid muscle, the descending hypoglossal branch of the ansa cervicalis, and the occipital artery may require division to mobilize the nerve for distal ICA exposure. Careful attention should also be given to the superior laryngeal nerve, which is usually located medial to the ICA. The superior laryngeal nerve divides into external and internal branches that pass posterior to the superior thyroid artery, and it may be harmed while controlling either of these two vessels. The glossopharyngeal nerve crosses the ICA near the base of the skull and is best protected by maintaining dissection very close to the anterior surface of the ICA. Excessive or prolonged retraction of the upper aspect of the incision may cause temporary compression injuries laterally to the greater auricular nerve or medially to the marginal mandibular branch of the facial nerve.
In patients with a high carotid bifurcation or an extensive lesion, mobilizing the ICA distally can be achieved by several maneuvers. The skin incision can be extended all the way up to the mastoid process, with complete mobilization of the sternocleidomastoid muscle toward its tendinous insertion on the mastoid process. It is important to avoid injury to the spinal accessory nerve, which enters the substance of the sternocleidomastoid muscle at that level.
The digastric muscle can be mobilized anteriorly, or if necessary, divided, given additional exposure (Fig. 27-1D). If further exposure is needed, the styloid process can be transected, and then the mandible can be displaced anteriorly. Some authorities have described dividing the ramus of the mandible to gain additional exposure.
The digastric muscle can be mobilized anteriorly, or if necessary, divided, given additional exposure (Fig. 27-1D). If further exposure is needed, the styloid process can be transected, and then the mandible can be displaced anteriorly. Some authorities have described dividing the ramus of the mandible to gain additional exposure.
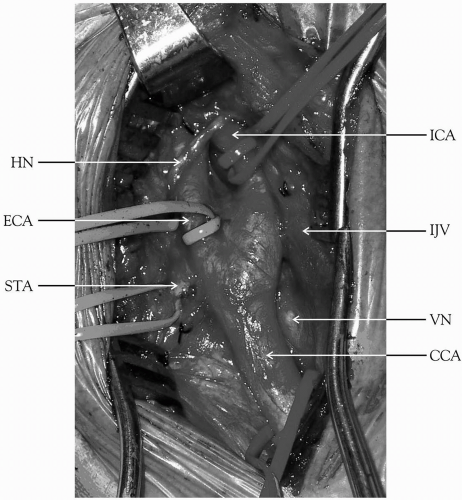 Figure 27-1. (Continued) E:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|