INDICATIONS/CONTRAINDICATIONS
With increasing availability of VATS, indications for an open lobectomy are evolving and obviously depend on the surgeons comfort level with VATS techniques. Table 15.1 demonstrates a framework for choosing an open approach in the VATS era. The decision to perform an open procedure can be made preoperatively or during an attempted VATS procedure. The limits of VATS technique vary by center and continue to evolve, mainly when dealing with more complex resections. The specific operator and center skills add an individual aspect to these indications. General advantages of an open approach are increased abilities to assess, control, and execute more complex resections, thus making it safer technically. There is increasing evidence that appropriate lymphadenectomy can be achieved by VATS even in complex situation such as postneoadjuvant treatments. Experts in robotic surgery argue that lymphadenectomy is more easily achieved by robotic surgery. It is our synthesis of the data and personal experience that VATS lobectomy for physiologically borderline patients is safer than an open approach. This challenges many of the common criteria upon which we judge medical operability for lobectomy. There is also some evidence to suggest that the prognosis of early stage NSCLC is superior following a VATS approach. Overall, we strongly believe that VATS lobectomy should gradually become the surgery of choice and the gold standard for early stage NSCLC and many benign indications. Correspondingly, an open approach should be reserved for specific cases. Other common indications for lobectomy include congenital, infectious, and inflammatory abnormalities and secondary malignancies.
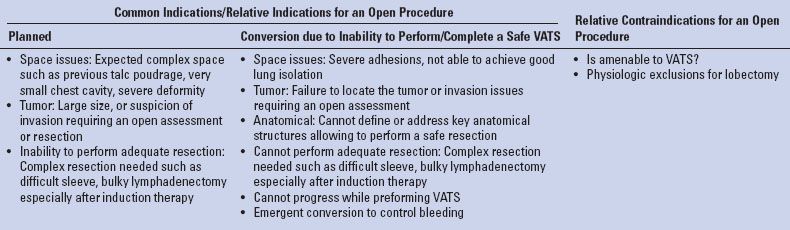
TABLE 15.1 Indications for Open Lobectomy in the VATS Era
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Diagnosis
It is recommended to strive for a tissue diagnosis before going ahead with a lobectomy. When tissue diagnosis has not been obtained in advance, in most cases there should be an attempt to perform a sublobar resection when feasible and proceed according to frozen section pathologic results.
Staging
When discussing primary lung cancer the T, N, and M stages should be defined well according to up-to-date guidelines (including brain imaging, PET etc.) prior to entering the operating room. The history and physical evaluation should be thorough, for instance, to look for specific clues such as pain, which can disclose metastasis or chest wall invasion, and to examine for cervical and supraclavicular nodes that can represent N3 disease. In the setting of left upper lobe (LUL) tumors any hoarseness should be investigated as suspicious for N2 disease. Imaging studies should be reviewed. As a rule of thumb, imaging should be current (<3 months old). Evaluate the need to repeat any test, especially in high-risk patients. Additional diagnostic studies, which can clarify and help plan the resection such as MRI for soft tissue or vascular invasion need to be considered. The need to perform mediastinoscopy, EBUS, or both, depending on the degree of concern for regionally advanced disease should be considered. It can be difficult to achieve sufficient sampling of stations 2 and 4 during left upper lobectomy. Moreover contralateral lymph node involvement is much more common with left-sided tumors making the need for invasive mediastinal staging even more essential.
Is the Disease Technically Resectable, and at What “Price”?
Prior to the procedure the imaging should be reviewed to assess the tumor, the important structures, and their relation to each other, as well as anatomical variants. Table 15.2 outlays some of the considerations to prevent or anticipate problems based on tumor imaging. An attempt to assess regional function (especially of the target zone) by hints such as high diaphragm, atelectasis, V/Q scans, etc. should be made. Information should be gathered from any previous intervention performed, especially involving the chest (e.g., previous OR report). Previous ipsilateral lung resection or mediastinoscopy may result in severe adhesions. Redo left chest surgery, inflammation, infection, destroyed lung and previous chemo or radiation might also cause severe adhesions resulting in a technically more challenging procedure, potentially more suitable for be performed open. If in doubt one can start with thoracoscopy either for assessing stage tumor invasion or as an attempted VATS lobectomy. When planning a bilateral resection, it is our practice to start with the limited resection and follow with the more extensive. This may improve the ability to oxygenate the patient throughout the procedure. If in doubt regarding the technical ability to perform the resection consider starting on this side since failure might exclude the possibility of further surgery.
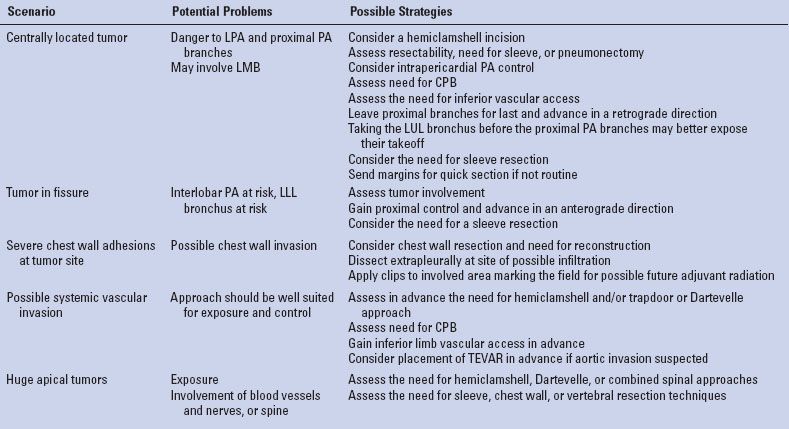
TABLE 15.2 Situations that Deserve Special Attention and Measures
Physiologic Competence
The ability to comfortably walk up two flights of stairs is very predictive of a patient easily able to tolerate a lobectomy from a respiratory point of view. Lung perfusion studies and cardiopulmonary exercise tests can further assess marginal patients. The potential need for pneumonectomy and the ability of the patient to tolerate it should be specifically evaluated. All other comorbidities should be assessed and optimized, such as a short course of steroids for COPD and smoking cessation. The need for an ICU bed and special postoperative issues such as mobility, social issues should be assessed in advance. There should be no major contraindications.
A thorough discussion with the patient/family detailing the risks and benefits of the proposed procedure should be held. The patient should be well educated about the procedure and perioperative period. There should be clear communication with Anesthesia, ICU, Nursing, and other support caregivers for best patient care. Providing best care is teamwork.
 SURGERY
SURGERY
Anatomical Considerations
Figure 15.1 provides a starting point to review the relevant anatomy of the left lung. The superior pulmonary vein (SPV) is an anterior mediastinal/hilar structure that forms as a confluence of the superior division of the LUL and the lingula. It generally has three to four divisions: The upper, which drains the apicoposterior and may further divide to apical and posterior; the middle, which drains the anterior and may further divide to three rami: Superior, inferior, and posterior; the third and fourth divisions, which drain the superior and inferior segments of the lingula (in 50% of patients, these are a single trunk). The lingular venous drainage might occasionally drain directly to the left atrium or to the inferior pulmonary vein (IPV). This may be revealed while dividing the anterior fissure. On rare occasions, the SPV and the IPV join within the lung or at the fissure to form a single trunk. Usually this configuration drains to the left atrium, but in rare occasions, it can have additional aberrant venous drainage(s). In one of four cases, the intrapericardial SPV and IPV will merge to form one venous trunk before entering the atrium. Figure 15.2 demonstrates the anterior view, including with intrapericardial exposure. The IPV is a posterior-inferior mediastinal structure. The IPV constitutes the upper border of the pulmonary ligament. Retracting the lung anteriorly will expose the posterior hilum and therefore will expose the IPV but not the SPV. Intrapericardially the proximal SPV is surrounded by a pericardial recess excluding its posterior aspect, which is more firmly attached to the fibrous pericardium. This recess can be entered to gain further venous length, more easily on the IPV but with dissection posteriorly also on the SPV. The back wall of the SPV is lying against the anterior wall of the left pulmonary artery (LPA) but slightly inferior. The proximal LPA branches are usually partially obscured by distal upper part of the SPV. Anteriorly the phrenic nerve runs on the medial side of the SPV from superior to inferior.
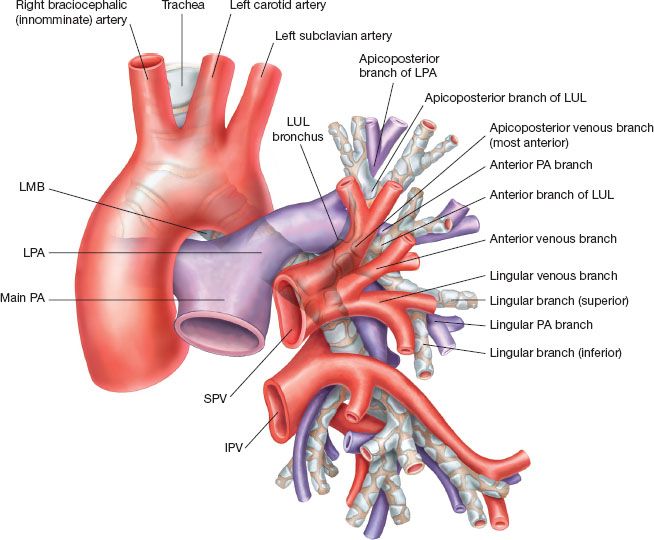
Figure 15.1 A schematic figure depicting the anatomical relations between the left bronchial, PA and SPV branches. Notice the direction of the PA to come above and around the LMB. Pay attention to the different PA branches as they bifurcate from the LPA. In the figure the PA branches directly from the LPA and runs from posterior to anterior as it follows the anterior segmental bronchus. However, alternate anatomy is possible where the PA anterior branches emerge as a common trunk.
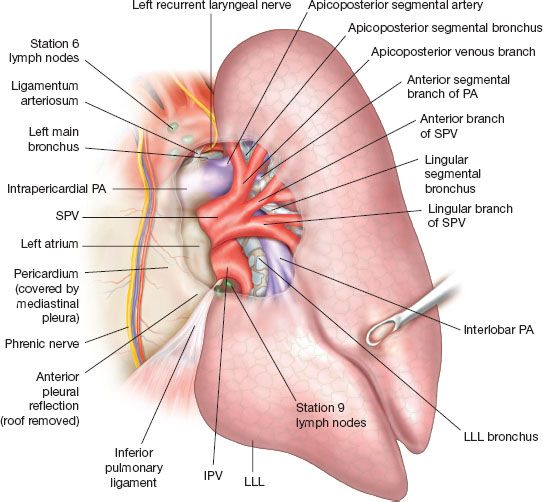
Figure 15.2 Anterior view while the lung is retracted posteriorly and laterally, showing the mediastinal intra- and extrapericardial relations of the PA, SPV, and IPV. Notice that the IPV is completely omitted beyond its proximal part, allowing for better understanding of the airway and PA anatomy. Pay attention to the ligamentum arteriosum and RLN.
The LPA emerges under the aortic arch anterior to the left main bronchus (LMB). As it exits the pericardium at its very proximal part, it is attached to the lower border of the aortic arch by a fibrous band, the ligamentum arteriosum roughly opposite the left subclavian artery takeoff. As the pulmonary artery (PA) continues distally it crosses over on top of the LMB to become posterior and lateral to it and then continues into the fissure in a lateral and anterior position in relation to the bronchial tree. At the posterior superior edge of the fissure the PA is solely covered by parietal pleura. Retracting the lung anteriorly and opening the pleura along the esophageal anterior wall will connect with the above, allow good exposure and usually an easy entrance point for a periarterial dissection of the PA.
The LPA gives off two groups of vessels: The truncus anterior equivalent, which is the proximal group (usually takes off before the PA crosses over the LMB) and the posterior arterial branches. The total number of branches varies from one to eight. Most patients (72%) will have three or four branches. The anterior group is composed of one to three vessels (70% have two branches and 15% have one or three). The posterior branches take off posteriorly and can be very short before entering the lung parenchyma (W). A prominent anterior trunk will suggest fewer posterior branches (W). As the PA enters the interlobar fissure it usually gives rise to blood supply of the superior segment of the left lower lobe (LLL)—the superior segmental artery, which can be composed of one to three separate branches (72%, 26%, 2%). Occasionally (13%), these vessels give rise to a posterior vessel crossing to the LUL. The lingular arteries usually originate distal to the superior segmental LLL artery(ies). However, in as much as 1/3 it will arise before the superior segmental takeoff(s). The lingular vessel (usually two either separate or branching from one trunk) gives rise to the entire lingular blood supply in 80%. In 10% crossing vessels from the basal segmental vessels to the lingula can be found and in 23.6% there is a lingular artery branching from the truncus anterior running behind the SPV. The interlobar PA can be found roughly at the center of the oblique fissure. It runs above and lateral to the bronchus. The bronchial bifurcation to the LUL and LLL is usually deep to the LPA in the fissure. The distal lingular PA branch will roughly mark the anterior border of the bronchial bifurcation.
Intrapericardially the superior and posterior borders of the LPA are attached to the parietal pericardium. Continuing medially the LPA is attached to the SPV by the ligament of Marshall (representing the remnant of the embryonic left superior vena cava), in rare occasions this could be a patent vessel and this structure may need to be divided to gain very proximal control of the LPA.
The vagus nerve usually crosses the upper border of the aorta between the carotid and the left subclavian arteries. It continues inferiorly just on the lateral aspect of the aortic arch to follow a posterior medial course along the esophagus. As it reaches the inferior border of the aortic arch it gives off the left recurrent laryngeal nerve just at the lateral border of the ligamentum arteriosum. The recurrent laryngeal nerve will encircle the aorta just posterior to the ligamentum arteriosum and ascend along the medial side to enter the tracheoesophageal groove. Stations 5 and 6 lymph nodes are close to the vagus/recurrent laryngeal and phrenic nerves. The nerves are usually well seen, being just immediately subpleural except for the recurrent laryngeal nerve. The lymph nodes are deeper to the phrenic and the vagus nerves; however, the course of the recurrent laryngeal nerve is at the bed of this dissection.
The LMB begins at the tracheobronchial angle, deep in the aortopulmonary window anterior to the pericardium. It bifurcates at an oblique angle from the trachea and continues under the aortic arch to a total length of 4 to 6 cm. The LPA passes anterior to the LMB and continues to cross above and posterior to it. The LUL bronchus is short and its total length is 0.5 to 1 cm as it bifurcates almost immediately to a medial inferior common bronchus (lingular) which descends and a lateral upper division common bronchus, which ascends. Retracting the lung anteriorly, the LMB is anterior to the descending aorta, above the IPV, and below and deeper to the LPA. The bifurcation between the LUL and the LLL bronchi is posterior to the SPV. From the anterior perspective it is better seen after dividing the SPV, lifting the distal SPV stump laterally and superiorly. From a posterior perspective, as seen in Figure 15.3, the LMB is best seen by retracting the lung anteriorly and looking below the PA.
Equipment
An open lobectomy requires a standard surgical tray. While performing a VATS lobectomy adequate equipment for open thoracotomy should still be available in case of an emergency conversion. Due to the nature of operating in a deep cavity we cannot overemphasize the need for a good headlight. Using the thoracoscopic equipment in an open procedure can facilitate lighting of the surgical field and can enable viewing difficult areas such as the apex the of the chest wall. We never pass off the thoracoscopic equipment after conversion to open thoracotomy and have available a separately wrapped flexible pleuroscope available during open cases.
Anesthesia and Preparation for Surgery
A team briefing is performed with the patient awake to review side, surgery, and preoperative medications. Lung isolation is preferably achieved by a double-lumen tube. We use a left-sided double-lumen tube except for left-sided proximal resections (sleeve lobectomy) and verify its position by a pediatric bronchoscope after intubation and after positioning the patient. Monitoring includes, at a minimum, an arterial line and a Foley catheter. All lobectomy cases have at least 2 units PRBC available on demand. For open procedures, we prefer to use epidural-based analgesia ± IV patient-controlled analgesia unless contraindicated. Safe epidural catheter placement is preferably done when the patient is awake, taking care that the taping of the catheter should be applied onto the contralateral side of the planned operation allowing for extension of the thoracotomy if needed. If contraindicated, intercostal blocks or insertion of a pleural catheter for continuous instillation of local anesthetic can be performed at the end of the case and supplement with post-op IV PCA. We routinely use a lower body warmer. A first generation cephalosporin is given as weight-adjusted prophylaxis, repeating every 4 hours or in the event of major blood loss. To prevent deep venous thromboembolism, our routine is to use preoperative dosing of subcutaneous heparin (5,000 units) 2 hours prior to attempted epidural placement, supplemented by sequential compression device stockings. A second “surgical time-out” is again held prior to skin cut to confirm side, operation, and operative plan. The anesthetic regimen is aimed at early extubation. If there is any concern regarding airway clearance bronchoscopy for toilet should be performed before extubating the patient. If needed, the endobronchial tube can be exchanged for a single-lumen tube allowing for better toilet using an adult-size bronchoscope or when prolonged mechanical ventilation is needed.
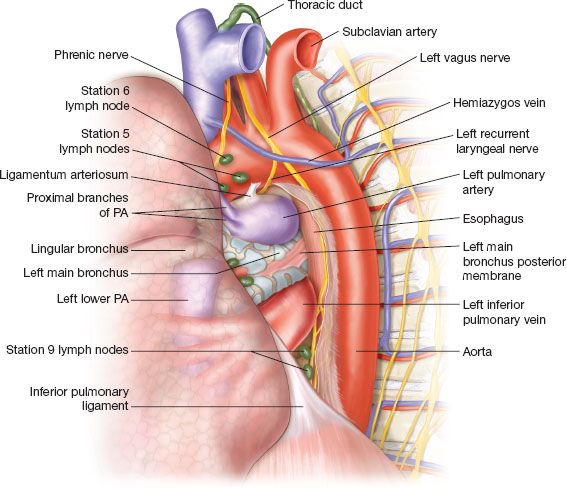
Figure 15.3 Posterior view of the posterior mediastinum and the left lung. The lung is retracted anteriorly. Notice the order of the PA, LMB, and IPV as they present from superior to inferior. Pay attention to the very proximal bifurcation of the first PA branches, which are prone to traction injury. As the LPA turns around the LMB to become superior and superficial to the PA, it lies in a subpleural position. This point is at the upper posterior edge of the left fissure and can be comfortably utilized to expose the PA and enter into the subadventitial plane.
Positioning and Incision
Posterolateral thoracotomy is our standard approach. We strive to perform a “semimuscle-sparing thoracotomy” by dividing latissimus but mobilizing the serratus anterior muscle from the chest wall. Making an effort to preserve the muscle allows better chest wall integrity and early perioperative shoulder function and it may become beneficial if a muscle flap will be considered; however, the rate of seromas is reported to be increased. Depending on the magnitude of the operation, the latissimus can either be retracted, divided, or mobilized for possible use as a major flap. The patient is positioned in the right lateral decubitus position, an axillary roll is placed just caudal to axilla to protect the brachial plexus, the table is flexed between the 12th rib and the upper pelvis, and reverse Trendelenburg positioning is used to flatten the flank and open the intercostal spaces. The patient is stabilized by a bean bag covered superficially by a gel pad and stiffened by applying suction. It should be hugging the patient bilaterally, away from the axilla and support the pelvis as well. All pressure points should be well padded. The sterile field should include the lateral border of the spine up to level of the neck allowing the extension of the thoracotomy posteriorly if required. At the anterior border it should include the sternum. Care must be taken to ensure the epidural catheter is not directly caught by the sterile covers or it can be inadvertently removed at the end of the case.
The chest is entered at the level of the fifth intercostal space. This correlates with the oblique fissure. For a better exposure one can shingle a posterior segment of the rib or completely resect a rib using the subperiosteal plane. This can facilitate entering a complicated pleural space such as when the lung is severely adherent to the chest wall. Occasionally starting with an extrapleural dissection can further facilitate this. If the use of an intercostal muscle flap is to be expected than harvesting the muscle should be done at this stage allowing safe handling and protecting its blood supply.
Anterolateral thoracotomy is an acceptable approach, yet limited for some complex resections. Alternative approaches include hemiclamshell incision for approaching large anterior tumors, the anterosuperior mediastinum, and the subclavian vessels. A trapdoor, Masaoka, or Dartevelle incisions allow for better access to the apex (positioning supine for all of these incisions)
After final positioning, verify that all lines, tubes, and devices are well placed and still working.
Intraoperative Assessment
Generally speaking, we approach most patients with either planned thoracotomy or planned VATS. However, thoracoscopy can be performed before going ahead with a thoracotomy. It is less invasive and may reveal findings encouraging for proceeding with VATS or alternatively may clearly mandate the need for an open procedure. On rare occasions it unfortunately might reveal advanced disease where lobectomy is not appropriate. Once thoracoscopy equipment is added, it can be helpful in an open approach for lighting and overcoming blind spots.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


