Transcatheter aortic valve implantation (TAVI) is an alternative therapy for symptomatic severe aortic valve stenosis in high-risk patients with several co-morbidities. We evaluated the 1-year effects of TAVI on quality of life, exercise capacity, neurohormonal activation, and myocardial hypertrophy. From June 2008 to October 2009, consecutive patients aged ≥75 years with symptomatic severe aortic valve stenosis (area <1 cm 2 ) and a logistic euroSCORE ≥15% or aged >60 years with additional specified risk factors underwent TAVI. An aortic valve prosthesis (CoreValve) was inserted in a retrograde fashion. Examinations were performed before and 30 days and 1 year after TAVI. An assessment of the quality of life (Minnesota Living with Heart Failure Questionnaire), a 6-minute walking test, measurement of B-type natriuretic peptide, and echocardiography were performed. In 51 patients (mean age 78 ± 6.6 years, mean left ventricular ejection fraction 58.4 ± 12.2%), the follow-up examinations were performed after TAVI. The 1-year follow-up visit after TAVI revealed significantly improved quality of life (baseline Minnesota Living with Heart Failure Questionnaire score 39.6 ± 19 vs 26.1 ± 18, p <0.001) and more distance covered in the 6-minute walking test (baseline 185 ± 106 vs 266 ± 118 m, p <0.001). The B-type natriuretic peptide level had decreased (baseline 642 ± 634 vs 323 ± 266 pg/ml, p <0.001), and the left ventricular mass index had decreased (156 ± 45 vs 130 ± 42 g/m 2 , p <0.001). The left ventricular diameter and ejection fraction remained unchanged. In conclusion, TAVI leads to significantly reduced neurohormonal activation, regression of myocardial hypertrophy, and lasting enhancement of quality of life and exercise capacity in patients with symptomatic and severe aortic stenosis 1 year after intervention.
The present prospective study was performed to determine the 1-year effects of transcatheter aortic valve implantation (TAVI) on patients’ quality of life and exercise capacity. In addition, we analyzed the changes in neurohormonal activation and myocardial function 1 year after TAVI.
Methods
From June 2008 to October 2009, consecutive patients with symptomatic severe and calcified aortic valve stenosis (aortic valve area ≤1 cm 2 ) and a risk precluding surgical aortic valve replacement were referred for TAVI. The risk of surgical aortic valve replacement was estimated using the logistic euroSCORE and the Society of Thoracic Surgeons Score. The indications and contraindications for TAVI in the present study have been previously described.
We performed a prospective single-arm study to assess the 1-year effects of TAVI on symptoms and myocardial function. The preliminary results were recently published. The local ethics committee approved the protocol. The patients gave written informed consent before participation. Examinations of the study patients were performed within 7 days before TAVI and 30 ± 7 days and 12 months ± 30 days after implantation.
Hypertension and hypercholesterolemia were diagnosed by medical history, medication use, and preprocedural measurements. The definition of hypertension was systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg, and the definition of hypercholesterolemia was blood cholesterol level >200 mg/dl). The estimated glomerular filtration rate was calculated using the abbreviated Modification of Diet in Renal Disease Study equation. Kidney disease was defined as a glomerular filtration rate of <60 ml/min/1.73 m 2 . Coronary artery disease was diagnosed in patients with a history of myocardial infarction, coronary artery bypass grafting, or previous percutaneous coronary intervention and in patients with stenosis of a coronary artery >50% on the preprocedural coronary angiogram.
TAVI was performed in 70 patients ( Figure 1 ). The aortic valve prosthesis (18-F-generation, Medtronic CoreValve Percutaneous System, Medtronic CV, Minneapolis, Minnesota) was inserted in retrograde fashion without hemodynamic support using a femoral arterial approach (n = 66) or a subclavian arterial approach (n = 4). Valves with 26- and 29-mm-expanded diameters were used. The 26-mm valve was selected if the aortic annulus was 20 to 23 mm (n = 23), and the 29-mm valve was selected for an aortic annulus of 24 to 27 mm (n = 47). After implantation, we performed additional dilation in 4 patients because of severe paravalvular regurgitation. This procedure led to central aortic regurgitation in 2 patients. Postprocedural aortography and transthoracic echocardiography immediately after TAVI were used to detect and grade aortic regurgitation. Immediately before and after implantation, invasive measurements were performed. Aortic and left ventricular pressures were recorded using 5Fr fluid-filled pigtail catheters connected to a pressure transducer. Invasive measurements included aortic systolic and diastolic pressure, peak systolic left ventricular pressure, and left ventricular end-diastolic pressure. Transaortic peak-to-peak pressure was calculated by subtraction of aortic systolic pressure from left ventricular systolic pressure. Each measurement was averaged for 5 cardiac cycles.
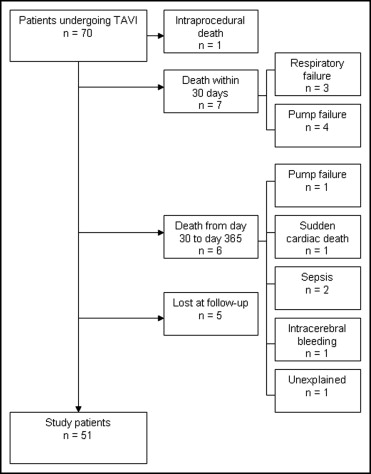
Within the first 30 days after implantation, 7 patients had died from respiratory failure (n = 3) or pump failure (n = 4). From day 30 to day 365, 6 patients died. Overall, 5 patients refused at least one of the ambulatory visits and were lost to follow-up. The remaining 51 patients formed the final study cohort ( Figure 1 ).
The Minnesota Living with Heart Failure Questionnaire (MLHFQ) was designed to measure the effects of heart failure and treatments of heart failure on the physical, emotional, social, and mental dimensions of quality of life. The summation of the responses to each item yields the total MLHFQ score for each patient. The test score ranges from 0 to 110, with a higher score indicating poorer quality of life. An adaptation of MLHFQ for German-speaking patients was used.
The 6-minute walk test was performed according to the guidelines of the American Thoracic Society. The patients were instructed to walk quickly for a period of 6 minutes or until dyspnea or muscular fatigue appeared. The walking course was 30 m long. The total walking distance was recorded.
Plasma B-type natriuretic peptide levels (normal value <130 pg/ml) were measured on the same day as the clinical investigations. The blood samples were collected in tubes containing ethylenediaminetetraacetic acid. After immediate centrifugation, B-type natriuretic peptide was measured using a chemiluminescent immunoassay kit (Biosite Triage, San Diego, California).
Echocardiographic studies were performed on the same day as the clinical investigations in all patients. Transthoracic echocardiography was performed according to the guidelines of the American Society of Echocardiography using a digital ultrasound scanner (Vivid 7, General Electrics, Horton, Norway). In the apical 5-chamber view, the peak and mean pressure gradients across the aortic valve were calculated using the Bernoulli equation. The effective aortic valve area was calculated using the continuity equation. Heart valve insufficiencies were evaluated according to the recommendations of the American Society of Echocardiography. Mitral and aortic regurgitation was graded as mild, moderate, or severe. If aortic regurgitation was present after TAVI, it was classified as transvalvular regurgitation or paravalvular regurgitation. When several aortic regurgitation jets were present after TAVI, aortic regurgitation was expressed as an overall grade. The measurement of aortic regurgitation after TAVI is difficult owing to the absence of standardized methods. We therefore used both, aortography and echocardiography, to assess postprocedural aortic regurgitation, with consistent results. The left ventricular end-systolic and end-diastolic diameter and posterior wall and septal thickness were measured by M-mode from the parasternal views. The left atrial volume was measured by manual tracing of the end-systolic endocardial borders using the apical 4-chamber view. The left ventricular myocardial mass was calculated according to the modified Devereux formula. The left ventricular ejection fraction was measured using Simpson’s method from the 4- and 2-chamber views.
A single observer performed the echocardiographic examination. Intraobserver variability was assessed in 10 patients by repeating the measurements on 2 occasions (1 to 5 days apart) under the same basal conditions. Variability was calculated as the mean percentage of error, derived as the difference between the 2 sets of measurements, divided by the mean of the observations.
The numeric values are expressed as the mean ± SD. The continuous variables were compared between patients before and after TAVI using the paired Student t test for normally distributed variables or the Wilcoxon test for non-normally distributed variables. The McNemar test was used to compare the categorical variables before and after TAVI. Box-and-whisker plots were generated to depict the MLHFQ score, walking distance in the 6-minute walk test, B-type natriuretic peptide level, and left ventricular mass index before and 30 days and 1 year after TAVI. All reported probability values are 2-tailed, and p <0.05 was considered statistically significant. Analyses were performed using the Statistical Package for Social Sciences statistical software package, version 17.0 (SPSS, Chicago, Illinois).
Results
The patients’ baseline characteristics are listed in Table 1 . The mean age was 78 ± 6.6 years. The mean log euroSCORE was 19.6 ± 11.3%, and the mean Society of Thoracic Surgeons score was 9.3 ± 4.8%. Immediately after percutaneous implantation of the aortic valve prosthesis, the left ventricular peak pressure and peak-to-peak pressure decreased, and the systolic blood pressure increased. The left ventricular end-diastolic pressure decreased slightly ( Table 2 ).
| Variable | Value |
|---|---|
| Age (years) | 78 ± 6.6 |
| Women | 26 (51%) |
| Log euroSCORE (%) | 19.6 ± 11.3 |
| Society of Thoracic Surgeons score (%) | 9.3 ± 4.8 |
| Body mass index (kg/m 2 ) | 28.1 ± 5.9 |
| Hypertension | 48 (94%) |
| Diabetes mellitus | 18 (35%) |
| Hypercholesterolemia | 29 (57%) |
| Coronary artery disease | 26 (51%) |
| Previous myocardial infarction | 13 (26%) |
| Previous percutaneous coronary intervention | 19 (37%) |
| Previous coronary artery bypass grafting | 7 (14%) |
| Atrial fibrillation | 10 (20%) |
| Previous stroke | 4 (8%) |
| Kidney disease | 33 (65%) |
| Chronic obstructive pulmonary disease | 19 (37%) |
| Variable | Before TAVI | After TAVI | p Value |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 114 ± 26 | 129 ± 27 | 0.005 |
| Diastolic blood pressure (mm Hg) | 51 ± 11 | 53.7 ± 10.8 | 0.386 |
| Left ventricular systolic pressure (mm Hg) | 181 ± 29 | 135 ± 27 | <0.001 |
| Left ventricular end-diastolic pressure (mm Hg) | 24.3 ± 7.5 | 22 ± 6.7 | 0.056 |
| Transaortic peak-to-peak pressure (mm Hg) | 66.1 ± 24.3 | 6.4 ± 5.5 | <0.001 |
Nonfatal pericardial tamponade occurred in 2 patients, and 2 patients experienced strokes. Vascular surgery (femoral vascular repair) was necessary in 6 patients. Vascular repair was also required in 2 patients in whom a subclavian artery approach had been used. Before TAVI, 8 patients required pacemaker stimulation. After TAVI, 10 patients had new complete heart block and 18 had relevant bradycardia associated with new-onset left bundle branch block. Therefore, 28 patients (55%) received a new permanent pacemaker. Aortic dissection, myocardial infarction, major bleeding, or renal failure requiring dialysis did not occur in any of the study patients.
Measurement of the New York Heart Association (NYHA) class, quality of life (MLHFQ), and B-type natriuretic peptide levels were performed in all study patients before and 30 days and 1 year after TAVI. Measurement of the distance in the 6-minute walk test was not feasible in 3 patients because of orthopedic or neurologic disease. Before TAVI, symptoms of heart failure owing to severe aortic valve stenosis occurred in all patients: NYHA class II in 3, NYHA class III in 41, and NYHA class IV in 7. At 30 days and 1 year after TAVI, the NYHA classes had improved ( Table 3 ). Moreover, at 30 days and 1 year after TAVI, the quality of life showed significant improvement ( Figure 2 ), and the distance covered in the 6-minute walk test had increased ( Figure 2 ). Additionally, the B-type natriuretic peptide had decreased ( Figure 3 and Table 3 ).
| Variable | Before TAVI | 30 Days After TAVI | One Year After TAVI |
|---|---|---|---|
| New York Heart Association functional class (III-IV) | 48 (94%) | 9 (18%) | 13 (26%) |
| p Value | <0.001 ⁎ | <0.001 † | |
| Quality of life ‡ | 39.6 ± 19 | 25.1 ± 17.7 | 26.1 ± 18 |
| p Value | <0.001 ⁎ | <0.001 † | |
| 6-Minute walk test (m) | 185 ± 106 | 248 ± 119 | 266 ± 118 |
| p Value | <0.001 ⁎ | <0.001 † | |
| B-type natriuretic peptide (pg/ml) | 642 ± 634 | 340 ± 253 | 323 ± 266 |
| p Value | <0.001 ⁎ | <0.001 † |
⁎ p Value for comparison of measurements before and 30 days after TAVI.
† p Value for comparison of measurements before and 1 year after TAVI.
In the echocardiographic examination, improvement of the hemodynamic properties was observed after TAVI. The aortic valve area had increased (baseline 0.84 ± 0.15 vs 1.96 ± 0.28 cm 2 at 1 year after TAVI, p <0.001), and aortic valve peak velocity had decreased (baseline 4.2 ± 0.6 vs 2 ± 0.36 m/s at 1 year after TAVI, p <0.001). Concomitant aortic valve regurgitation was common before TAVI (n = 40, 78%). In the follow-up examination performed after 1 year, aortic regurgitation showed a tendency toward a lower degrees of severity (moderate aortic valve regurgitation before TAVI, n = 11 vs moderate aortic valve regurgitation 1 year after TAVI, n = 4, p = 0.052). However, the character of aortic valve regurgitation varied. We observed mild central aortic regurgitation in 2 patients and mild or moderate paravalvular aortic regurgitation in 21 patients. The mild central aortic regurgitation resulted from additional dilation after TAVI owing to severe paravalvular regurgitation. Severe aortic regurgitation did not occur after TAVI in our study patients.
The left ventricular diameter and volume remained nearly constant after TAVI. Changes in left ventricular ejection fraction, left atrial volume, and concomitant mitral valve regurgitation are listed in Table 4 . The intraobserver variability for the echocardiographic measurements was 4%.



