Off-Pump Coronary Artery Bypass Grafting
Bobby Yanagawa
John D. Puskas
Introduction
Myocardial revascularization began with the sentinel association between angina and myocardial ischemia. This led to the development of procedures aimed at restoring or augmenting blood flow to the heart including epicardial abrasion, pectoralis-to-myocardial muscle flaps, left internal mammary implantation, and ultimately the coronary artery bypass graft (CABG) procedure. Despite dramatic improvements in coronary stent technology and medical therapy including anticoagulants and lipid-lowering therapies, CABG is still the gold standard intervention for complex coronary anatomy including those with left main disease, multivessel disease as well as patients with left ventricular dysfunction. More recently, CABG has firmly demonstrated improved survival versus percutaneous coronary intervention (PCI) for patients with multivessel coronary disease and diabetes mellitus, sparking a renewed interest in surgical revascularization.
The contemporary challenge in surgical revascularization is to leverage the long-term patency of bypass grafts while reducing the incidence of perioperative complications, in particular, stroke. The potential causes of perioperative stroke can be conceptualized as low cerebral perfusion, especially in association with intrinsic cerebrovascular disease, and micro- and macroembolism. The major sources of emboli are microemboli from the use of cardiopulmonary bypass (CPB) and atherosclerotic emboli from manipulation of the ascending aorta. Off-pump CABG (OPCAB) has been proposed to address these limitations of surgical revascularization.
Since Vasilii Kolesov (1967) first published his series of LIMA to left anterior descending (LAD) anastomosis without the use of CPB, OPCAB has been adopted in select centers worldwide. Today, a minority of CABG procedures are performed off pump and this is due to greater technical difficulty, the lack of clear mortality benefit in large randomized controlled trials, and the suggestion of poorer patency outcomes in some series.
Mastery of OPCAB requires a focused and sustained effort to master a new set of physical and psychological skills. If precise and complete revascularization can be accomplished by OPCAB, then the morbidity attributable to aortic cannulation, clamping, cardioplegia, and the use of CPB will be avoided and the patient should benefit.
The single absolute indication for OPCAB is to facilitate surgical revascularization in a patient with a severely atherosclerotic aorta, also referred to as porcelain aorta. Here, violation of the ascending aorta for cannulation, cross clamp, or for construction of proximal anastomosis is associated with high risk of embolism and stroke. OPCAB can be used to accomplish a completely no-touch aorta technique. For this reason, every institution performing surgical revascularization should have at least one surgeon who can perform OPCAB in an emergent situation or in the case of severe aortic plaque.
In addition, there are small randomized controlled trials and a large volume of restrospective database evidence to suggest more favorable outcomes for OPCAB in patients presenting with the following risk factors:
Acute/subacute STEMI
Female
Repeat revascularization
Dialysis-dependent renal failure
Advanced age
Previous stroke/cerebrovascular disease
Elevated the Society of Thoracic Surgeons (STS) predicted risk score
Vulnerable, high-risk patient cohorts may benefit from avoidance of myocardial stunning, hypothermia, formation of microemboli, and systemic inflammation associated with CPB. In patients with previous stroke and the elderly, avoidance of aortic manipulation may reduce stroke risk. Patients with acute coronary syndrome may benefit from preservation of coronary flow and avoidance of myocardial stunning associated with CPB. As always, good surgical judgment based on individual patient risk factors and surgeon experience must guide decision making.
OPCAB is contraindicated in patients who are hemodynamically unstable. For instance, patients in refractory cardiogenic shock should be stabilized on CPB to unload the ventricle, lowering the workload, and decreasing the oxygen demand of an acutely ischemic failing heart.
There is no question that OPCAB is more technically difficult than on-pump CABG. This technique requires greater judgment of optimal grafting strategy, OPCAB candidacy, and coordination between anesthetist and surgeon. Some patient-related factors can make this procedure very challenging:
Multiple difficult lateral wall targets
Cardiomegaly
Severe left ventricular dysfunction
Intramyocardial coronary arteries, especially in high lateral wall targets
Small or diffusely diseased coronary arteries
Anticipated need for endarterectomy or plasty
Mild to moderate aortic or mitral regurgitation
Preoperative hemodynamic instability
Pulmonary hypertension
Urgent/emergent cases
Left main coronary artery disease
Ischemic arrhythmia
Pectus excavatum, other chest wall deformity, or prior left pneumonectomy that displaces the heart into the left chest
Persisting in a challenging OPCAB can lead to urgent or emergent conversion to CPB. In our experience, patients more likely to require conversion from an initial OPCAB
strategy to CPB include those with ischemic arrhythmias, those with severe LV dysfunction and cardiomegaly, those with deep intramyocardial coronary artery targets, and those needing coronary endarterectomy. Emergent conversion to CPB has been associated with poor outcomes in multiple published series. Depending on the experience of the surgical team, such patients may be better served with an on-pump procedure.
strategy to CPB include those with ischemic arrhythmias, those with severe LV dysfunction and cardiomegaly, those with deep intramyocardial coronary artery targets, and those needing coronary endarterectomy. Emergent conversion to CPB has been associated with poor outcomes in multiple published series. Depending on the experience of the surgical team, such patients may be better served with an on-pump procedure.
Surgeon and Team Experience
The importance of the learning curve is illustrated by the differing outcomes of two contemporary randomized controlled trials: ROOBY and CORONARY. ROOBY required a minimum experience of 20 OPCAB cases and residents were permitted to be the primary surgeon in a majority of cases. The CORONARY trial required surgeons to have >100 cases or 2 years experience. ROOBY was associated with higher rates of on-pump conversion (12% vs. 8%), incomplete revascularization (17% vs. 12%), and repeat revascularization (6% vs. 1.4%). Given this data and our experience with OPCAB, it seems clear that the learning curve for OPCAB extends well beyond 20 cases. Mastery of OPCAB requires mentorship with an experienced OPCAB surgeon and at least 100 independent cases. Even after >3,000 OPCAB cases (JDP), we still find new challenges and are continually learning and adapting our technique.
In our center, our trainees learn the technical points of the OPCAB anastomosis on the anterior distal target initially, progressing to more difficult inferior and lateral wall targets. Alternatively, surgeons can gain experience and confidence with OPCAB skills by performing on-pump beating heart bypass using cardiac positioning and suctioning devices. Finally, an experienced anesthesiologist is critical to the OPCAB team to maintain hemodynamic stability while optimizing surgical exposure to perform the surgical anastomoses. Indeed, experience of the overall surgical team is needed to optimize coordination of the surgeon, anesthesia, perfusion, nursing, and assistant to safely and reproducibly perform precise and safe OPCAB.
Anesthetic Considerations
In addition to routine intraoperative monitoring, transesophageal echocardiography is recommended for assessment of ventricular function and any valvulopathy. A perfusionist should be in the room for the duration of the procedure. In higher-risk cases, we routinely prime the CPB circuit.
Arterial pressure is maintained with a combination of autotransfusion using Trendelenburg positioning, administration of intravenous fluid, and with vasopressor or inotrope support. The use of Trendelenburg position can provide dramatic volume from autotransfusion of intravascular volume and should be the first maneuver to increase preload. Reverse Trendelenburg can be helpful in lowering blood pressure for the proximal anastomosis to facilitate partial cross clamp or proximal anastomosis device. Alpha-adrenergic vasoconstricting agents (e.g., norepinephrine) can support the arterial pressure during the distal anastomosis and are preferred over inotropic support (e.g., epinephrine) which will increase cardiac contraction making an anastomosis more technically challenging and increasing myocardial oxygen demand. Sodium nitroprusside may be used for vasodilation, especially during proximal anastomoses.
An experienced anesthesiologist should recognize subtle changes in hemodynamic status, including gradual elevation in pulmonary artery pressures, increased requirement of inotropes and vasopressors, and especially arrhythmias that can be a sign of impending cardiovascular collapse. Myocardial ischemia upon arteriotomy or occlusion of a target vessel may be resolved with an appropriately sized intracoronary shunt or may require rapid conversion to CPB. Again, protracted hypotension, shock, and malignant arrhythmias should prompt rapid conversion to CPB.
The patient must be kept normothermic to avoid platelet dysfunction and ventricular arrhythmias. Without the heat exchanger of the bypass circuit to regulate the body temperature, ancillary methods are necessary. This can be accomplished with the use of infusing intravenous fluids through warmers, warming inhalational anesthetic agents, maintaining warm room temperatures, and by the use of a convective forced-air warming Bair Hugger system (Arizant Healthcare, Eden Prairie, MN).
Anticoagulation regimens vary according to surgeon preference. We use an initial half dose of Heparin IV (1.5 mg/kg) 3 minutes before the internal mammary artery (IMA) is divided and maintain a continuous infusion of heparin 6,000 units/hour to keep the activated clotting time (ACT) >300 seconds. Reversal of anticoagulation is accomplished with a dose of protamine after the last anastomosis calculated to return to the ACT to a normal baseline level. Some surgeons advocate a full pump dose of heparin, particularly if the risk of conversion to CPB is high.
We do not stop aspirin preoperatively, and give 325 mg PO on the night before surgery; another 325 mg is given per rectum as the Foley catheter is inserted in the operating room.
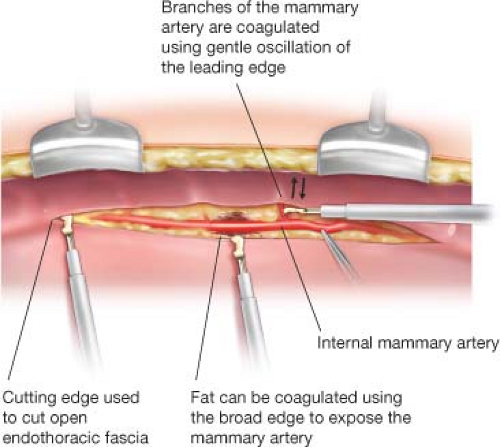
Median sternotomy is performed and the IMAs are harvested as skeletonized grafts using a Harmonic scalpel (Harmonic Synergy, Ethicon, Somerville, NJ). Skeletonized harvest of IMA grafts minimizes sternal injury and thus potential for pain and sternal wound infection and allows for the greatest graft length. Moreover, the exposed skeletonized mammary artery facilitates sequential anastomoses. The Harmonic scalpel uses high-frequency mechanical vibration to cut and coagulate tissues (Fig. 26.1). The mammary branches can be coagulated by gently oscillating the broad edge of the scalpel against the branch. Compared with electrocautery, the Harmonic scalpel minimizes the risk of injury to the adjacent mammary and sternum during harvest. Radial artery and occasionally saphenous vein conduits are harvested endoscopically, simultaneous with skeletonized IMA harvest.
Assessment of the Aorta
Comprehensive assessment of the aorta should be performed preoperatively and intraoperatively. This is critical to reduce the risk of aortic atheroembolism. Aortic calcification can be appreciated on chest x-ray, cardiac catheterization, and CT scan. We
routinely obtain a CT for patients at high risk for aortic atherosclerosis such as those with peripheral vascular disease, history of stroke, ostial left main disease and smoking history, or any evidence of aortic calcification on the aforementioned studies.
routinely obtain a CT for patients at high risk for aortic atherosclerosis such as those with peripheral vascular disease, history of stroke, ostial left main disease and smoking history, or any evidence of aortic calcification on the aforementioned studies.
TABLE 26.1 Epiaortic Ultrasound Grading Scale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Intraoperative assessment of the aorta includes transesophageal echocardiography, manual palpation, and epiaortic ultrasonography. We utilize epiaortic ultrasound routinely for all cardiac surgery patients. The procedure takes only 1 to 2 minutes and provides an easy, noninvasive, and sensitive method for assessing the extent of plaque burden disease in the ascending aorta. In a large retrospective series, its use has been shown to impact surgical decision making in 4% of cases. It is particularly valuable for identification of soft, noncalcified plaque which cannot be appreciated by palpation and without calcification, cannot be seen on preoperative imaging. The grading scale of aortic atherosclerosis is shown in Table 26.1.
Cardiac Positioning and Stabilization
Exposure is the key to OPCAB. Patience is needed to achieve the optimal exposure while ensuring hemodynamic stability. In our experience, most hemodynamic problems during lateral wall grafting are due to imperfect cardiac positioning, not ischemia. Thus, we strongly advise surgeons to displace the heart slowly, gently, incrementally, and persistently. Furthermore, manipulation of the heart should be done with suction devices, traction sutures, table rotation and tilt, and gravity. Surgeons should resist the temptation to manipulate the heart with their hands.
The following are key maneuvers for OPCAB exposure:
Wide pericardiotomy, freeing the left pericardium from the diaphragm
Elevation of the right sternal edge with rolled towels
Freeing of right pericardium from diaphragm (open right pleura for very large hearts, low ejection fraction, bilateral IMA harvest)
Single deep pericardial traction “LIMA” suture between inferior vena cava and left inferior pulmonary vein
Loosening right pericardial traction sutures
Avoidance of compression of the heart against the right pericardium, sternum, or retractor
As a general rule, the heart tolerates rotation but not compression. The left pleura is opened to facilitate LIMA harvest and to allow the left pericardium to move freely in response to tension on traction sutures. The pericardium is incised in an inverted T-configuration, and then incised laterally along the diaphragm. The left lateral pericardial incision is extended to the apex, which is essential to allow the pericardium to be fully retracted, displacing the heart and exposing the lateral wall of the left ventricle. The left pericardium is incised vertically (while preserving the left phrenic nerve) at the level of the pulmonary artery to allow the LIMA a straight line toward the LAD target. The right pericardium is also dissected along the diaphragm and the right pleural space can be opened to facilitate RIMA harvest and to allow the heart to fall into the right chest during lateral displacement.
The depth and location of the pericardial retraction sutures are critical for exposure and lateral displacement of the heart. An important traction suture is the deep “LIMA stitch” (first described by Ricardo Lima, MD), placed approximately two-thirds of the way




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree