Fig 1.
Extraparenchymal mass forms an obtuse angle with the chest wall in a patient with a solitary plasmacytoma. Note the bone destruction indicating the origin of the tumor outside the lung and pleura
Chest Wall Disease
The normal chest wall is symmetric. The most common causes of asymmetry are rotation and kyphoscoliosis. On chest radiography, the side to which the patient is rotated is more radiolucent (in a left anterior oblique/right posterior oblique position the right side is more lucent). Chest radiography is useful to evaluate skeletal disease, calcifications, and subcutaneous emphysema.
Calcifications
Calcifications deposited in soft tissues may be metastatic or dystrophic. Metastatic calcifications occur with elevated serum calcium, in which case calcium hydroxy-apatite crystals are deposited in multiple locations, including the lung, stomach, kidney, and vascular system [1]. Dystrophic calcifications in altered necrotic, or dead tissues are seen in patients with normal serum calcium and phosphorus levels and occur in association with connective tissue disorders, in subcutaneous tissues, muscles, and fascial planes as calcification universalis.
Soft-Tissue Masses
These must first be differentiated from asymmetries arising from patient positioning, contralateral surgery (mastectomy), and atrophy (poliomyelitis and stroke). Primary tumors are rare. Malignancies include fibrosarcoma and malignant fibrohistiocytoma, with lipomas as the most common benign primary tumor. Neurogenic tumors, such as neurofibromas, arise from intercostal nerves or the paraspinal ganglia and can be found at multiple sites in neurofibromatosis type 1 (von Recklinghausen disease).
Inflammatory Diseases of the Chest Wall
Bacterial infection of the chest wall is rare and typically involves the ribs and sternum. Osteomyelitis can spread hematogenously or directly from an adjacent infectious process, typically Staphylococcus aureus or Pseudomonas aeruginosa. Radiography may demonstrate soft-tissue swelling overlying rib destruction and a periosteal reaction in chronic cases but computed tomography (CT) and MRI are much more sensitive and specific. CT is limited in its detection of bone marrow abnormalities, while MRI allows the early detection of osteomyelitis by showing bone marrow edema on T2 and marrow hypointensity on T1 images. However, CT is more sensitive than MRI in showing cortical destruction and a periosteal reaction.
Chest wall involvement with tuberculosis (TB) is uncommon, occurring via hematogenous spread or, more rarely, by direct extension from the underlying lung or pleura; the latter is referred to as empyema necessitans. Chest wall involvement in TB manifests as osseous and cartilaginous destruction and soft-tissue masses with calcifications and rim enhancement [2]. Fungal infections of the chest wall occur in immunocompromised patients, with Aspergillus accounting for 80–90% of cases [3]. In 15% of the cases of thoracic actinomycosis, the fungus invades the chest wall, creating fistulas and empyema necessitans. Postoperative patients are at risk of Nocardia infection.
Congenital Diseases of the Chest Wall
Poland syndrome is characterized by aplasia of the pectoralis major muscles, and occasionally by hypoplasia of the ipsilateral chest wall, scapula, and ribs. Imaging reveals a smaller and more lucent hemithorax, while cross sectional imaging demonstrates the absence of the pectoral muscles and a smaller, hypoplastic hemithorax [4].
Pectus Excavatum
In this variant in chest wall development (incidence 1/400–1/1000), the sternum is depressed relative to the anterior chest (ribs) and often tilts rightward, with the mediastinum leftward [3, 5]. In most cases, it is a cosmetic issue only, but in severe cases, it can lead to pain, dyspnea, and restrictive lung disease. On radiographs, the right side of the mediastinum is indistinct, simulating middle lobe airspace disease. The depressed sternum is visible on the lateral projection [3].
Injuries to the Thoracic Skeleton, Rib Fractures, and Trauma
Upper rib fractures suggest severe trauma, and a search should be made for associated injury of the aorta, great vessels, and brachial plexus [6]. Fractures of the medial clavicle and sternum are also associated with vascular and cardiac injury. Sternal fractures are very difficult to visualize on frontal chest radiographs and may be seen on the lateral view, but nearly all are visible by CT, which will demonstrate any adjacent hematoma. Significantly displaced sternal fractures should lead one to look for associated cardiac injury. Lower rib fractures can be associated with hepatic, renal, or splenic injury. Abdominal radiographs are helpful to depict fractures of the lower ribs, as they are better visualized.
A flail chest (or segment) occurs with five or more consecutive rib fractures (or three or more ribs fractured in two places). The flail segment moves paradoxically during respiration, with resultant ventilatory compromise. Fractures of the thoracic spine account for 15–30% of all spinal fractures. The most vulnerable segment is at the thoracolumbar junction (T9–T12). Most (∼70%) thoracic spine fractures are visible on radiographs, but CT shows almost all of them and, more importantly, will demonstrate the displacement or retropulsion of bone fragments into the spinal canal and cord.
Bone Masses
The most common non-neoplastic tumor of the thoracic skeleton is fibrous dysplasia, which accounts for 30% of the benign bone tumors of the chest wall [7]. These slow-growing tumors often occur in the posterolateral ribs and are usually asymptomatic unless pressure symptoms or pathologic fractures occur. Fibrous dysplasia manifests as an expansile lytic lesion with a hazy or ground glass appearance. Plasmacytoma and multiple myeloma are the most common malignant neoplasms of the thoracic skeleton and can present as extraparenchymal masses with bone destruction, similar in appearances to other metastases [8]. Bone lesions are well defined; they have a punched out appearance. In advanced cases there is marked erosion as well as areas of expansion and destruction of bone cortex, sometimes with thick ridging around the periphery, giving a “soap bubble” appearance.
Thalassemia
With extramedullary hematopoiesis, hematopoietic tissues outside the bone marrow produce blood cells. This occurs mostly in the chronic hemolytic anemias, with thalassemia being the most common. Normal marrow tissue can expand outside the medulla through permeative erosions or by the reactivation of previously dormant hematopoietic tissues [9]. Ineffective erythropoiesis leads to expansion of the bone marrow space in the ribs, long bones, vertebral column, skull, and, characteristically, facial bones, and may lead to osteoporosis. Paravertebral and rib lesions are most often seen incidentally but can occasionally cause mass effect on the spinal canal and cord compression. On radiography, bilateral lobulated paravertebral masses and rib expansion are seen.
Pleural Disease
When assessing for pleural effusion or pneumothorax, the patient’s positioning for the radiograph should be taken into account, as air moves upwards and fluid moves downwards within the pleural space. In the critically ill supine patient, air will rise to be anterior and fluid will layer posteriorly and over the apex as a cap. Intraperitoneal gas rises to the highest point, under the diaphragm dome on upright radiographs. Even ill patients can roll onto their side for a decubitus view; in those with effusions place the effusion side down to allow the fluid to layer along the ribs. For suspected pneumothorax, the abnormal side should be up, to allow the air to rise.
Pleural Effusion
On upright studies, pleural fluid produces a meniscus sign, displacing the affected lung away from the costophrenic sulci and blunting the angle. Evaluate the posterior costophrenic sulcus (the lowest point) on upright chest radiographs for small pleural effusions. On the frontal view, evaluate the lateral costophrenic angle or sulcus, keeping in mind that a subpulmonic effusion may be present with the lateral costophrenic angle unblunted. With subpulmonic fluid the ipsilateral hemidiaphragm will be of uniform attenuation. There will be a lack of visibility of the posterior lung vessels through the hemidiaphragm on the frontal view, and lateral displacement of the apparent dome of the hemidiaphragm (with a more sharply sloping lateral aspect). On supine radiographs, free flowing pleural fluid layers posteriorly, possibly obscuring the meniscus or borders (with lung or normal pleura). If the effusion is large enough, there may be an apical pleural cap, as fluid caps the apex of the lung.
Pleural fluid may be loculated in the presence of adhesions or scars. It can track into fissures even if not loculated and is often recognizable because of its tapering cigar-shaped appearance. A “pseudotumor” can occur when the fluid has a more rounded shape; a homogeneous density with a different shape on two orthogonal projections is a clue to its nature (as is a comparison between old and follow up radiographs showing a change in size).
With large pleural effusions, mediastinal structures will deviate to the opposite side. Another important effect of large pleural effusions is diaphragmatic inversion. When this occurs, the two hemidiaphragms move in opposite directions during respiration. As a result, air may move over and back between the two lungs (pendelluft or pendulum respiration), with the net effect being increased dead space and significant dyspnea. Thoracentesis with removal of enough fluid to restore the affected hemidiaphragm to its normal position may provide marked relief of symptoms. Left hemidiaphragm inversion is easier to diagnose, since it results in mass effect in the left upper quadrant, displacing the gas-filled stomach.
In hospitalized patients, pleural effusions may arise as a consequence of congestive cardiac failure or post-surgically. Congestive heart failure effusions are usually bilateral and typically symmetric, although occasionally they are unilateral or more asymmetric, with the right larger than the left. With unilateral effusions (or left larger than the right) in a non-surgical patient, other causes include pneumonia, infarction, neoplasm, trauma, and connective tissue disorders (especially systemic lupus erythematosus and rheumatoid arthritis). With pulmonary embolus, radiographs may reveal small unilateral or bilateral hemorrhagic pleural effusions and atelectasis.
Adjacent upper abdominal abnormalities can cause effusion (most often unilateral), with the most common etiologies including splenic trauma, subdiaphragmatic abscess, pancreatitis, and ascites. Here, the clinical history is very helpful, while thoracentesis can be diagnostic (e.g., elevated amylase with pancreatitis). With ascites (e.g., due to liver failure), pleural effusions can develop because of ascites leaking through the diaphragm. When a right subdiaphragmatic abscess is suspected ultrasound can be very helpful, but for suspected left upper quadrant fluid collections CT is better.
Trauma can result in pleural effusion, more commonly unilateral. This is obvious with multiple rib fractures but less apparent with, for example, aortic laceration in the absence of fractures. Another less obvious case is a malpositioned vascular catheter or feeding tube. Inadvertent placement of a feeding tube into the lung is usually benign, as long as it is discovered before a feeding is given. However, pleural placement is not similarly benign, as tension pneumothorax and empyema are among its important consequences.
Empyema
Pneumonia patients commonly have uninfected pleural fluid (sympathetic effusion). Infection of the pleura (empyema) can occur as a complication, with organisms such as TB and fungi having a particular predilection for the pleural space. The differentiation of empyema from lung abscess can generally be accomplished by chest X-ray alone and corroborated by thoracic ultrasound. Empyemas typically make right or obtuse angles with the adjacent chest wall, while lung abscesses usually demonstrate acute angles only. In addition, empyemas are usually oval or lenticular in shape and therefore are larger on one of two orthogonal projections, while lung abscesses are more spherical in shape and thus more similar in size on two orthogonal projections. CT can help by demonstrating the empyema as more mass-like, deflecting vessels and bronchi in its path, while lung abscess is more destructive of lung structures but less mass-like. The smoother margins of a pleural abnormality and the CT “split pleura sign” of enhancing visceral and parietal pleura around an empyema can also aid in the differential diagnosis (Fig. 2).
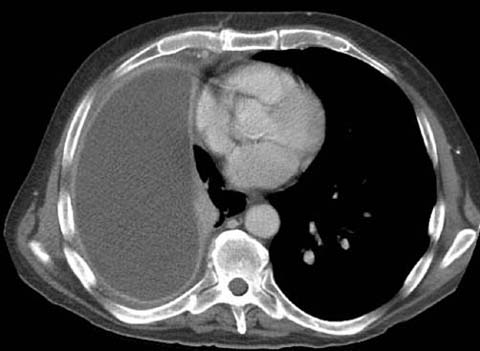
Fig 2.
Right pleural effusion with thickened visceral and parietal pleura (“split pleura sign”)
Neither chest X-ray nor CT alone can establish the presence or absence of infection in the lung or pleural space; rather, clinical findings (such as fever and elevated white blood cell count) are important in raising the possibility of infection. The role of imaging is to localize the infection to the lung or pleura. The final etiology of pleural effusion is sometimes established on clinical grounds alone. In difficult cases, diagnosis generally relies on thoracentesis and an analysis of the pleural fluid. Ultrasound imaging and marking of the adjacent chest wall can be helpful to guide thoracentesis.
Pneumothorax
Air can enter the pleural space as a result of lung, tracheobronchial or esophageal injury. Pneumothorax is seen in 15–40% of acute chest trauma patients. Upright frontal radiography is preferred for detecting pneumothorax, as air tends to rise up within the pleural space. However, lateral decubitus radiography with the suspected side of the pneumothorax up is an alternative. Many small and even moderate-sized pneumothoraces not visible on supine radiographs are readily identified at CT.
Pneumothorax is best diagnosed by visualization of the lung edge outlined by pleural air. Apical lucency and absence of vessels are less reliable and can be produced by bullae. As for pleural effusions, the detection of pneumothorax becomes difficult with supine patients, with the non-dependent portion of the pleural space near the anterior hemidiaphragm. Lucency near the lung bases should raise suspicion, particularly in the presence of the deep sulcus sign, even when no lung edge is visualized. Pneumothorax can track into fissures with air in the minor fissure considered as another sign of supine pneumothorax.
A tension pneumothorax is a medical emergency, as mediastinal structures can be compressed, causing decreased venous return to the heart and hemodynamic instability. With increasing intrapleural pressure, mediastinal structures deviate contralaterally, with ipsilateral hemithorax hyperexpansion and hemidiaphragm depression.
The differential diagnosis for pneumothorax is far less extensive than that for pleural effusion. Most cases follow trauma but can occur secondary to ruptured blebs, with the latter typically found in aesthenic young males. Interstitial lung disease is another potential cause and honeycombing or lung cysts increase the risk of pneumothorax, particularly in males with eosinophilic granuloma and in females with lymphangioleiomyomatosis.
Other potential causes, in which the radiograph is normal, include subpleural abnormalities that tend to cavitate, such as tuberculosis and metastases. Squamous primary neoplasms account for most cavitary metastases, but cavitation is also a feature of sarcomas. Increased intrathoracic pressure (e.g., asthma or pregnancy) is another potential explanation for pneumothorax with an otherwise normal or nearly normal radiograph. A persistent pneumothorax after chest tube insertion and suction suggests either a malfunctioning chest tube, bronchial injury, or bronchopleural fistula.
A special category of pneumothorax is the pneumothorax ex vacuo, which is a complication of lobar collapse [10]. In this condition, the collapse results in a sudden increase in negative intrapleural pressure surrounding the collapsed lobe. As a result, gas from the ambient tissues and blood is drawn into the pleural space while the seal between the visceral and parietal pleura of the adjacent lobe or lobes remains intact. The pneumothorax resolves as soon as the bronchial obstruction is relieved and the collapsed lobe re-expands. Recognition of this entity is critical as appropriate treatment is to relieve the bronchial obstruction rather than insert a pleural drainage catheter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


