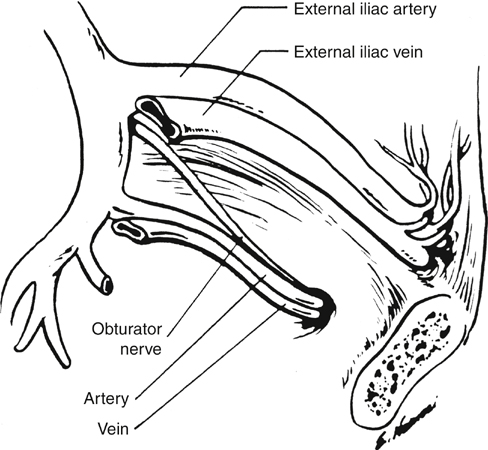
Routing of an arterial bypass graft from the retroperitoneum into the leg through the obturator foramen was introduced by Shaw and Baue in 1963. Although the technical details of the procedure have been modified over the last nearly 5 decades, its fundamental rationale has remained the same, namely, to provide inflow to the extremity while excluding the new graft from communication with the femoral vessels in the groin. Similarly, although the indications for obturator foramen bypass have broadened somewhat, the procedure is most often used to manage groin sepsis caused by an infected previous graft, an infected false or true femoral aneurysm, or some other suppurative process (Box 1). Safe construction of an obturator bypass requires familiarity with anatomic planes not often encountered by the vascular surgeon. In brief, the obturator nerve and the obturator artery and vein pass through the superior and lateral aspect of the obturator membrane and are the key landmarks in identifying the foramen. Although the obturator artery arises from the anterior division of the internal iliac artery in most people (Figure 1), in nearly 30% of patients it arises entirely, or in part, from the inferior epigastric artery. In this case, the vessel courses posteriorly over the superior ramus of the pubis, often accompanied by a small vein, and is vulnerable to injury if not carefully identified. The prominent obturator internus muscle overlies the inferior aspect of the foramen.
Obturator Foramen Bypass Grafts in Groin Sepsis
Operative Procedure: Anatomic Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine