Chapter 62 Obesity Hypoventilation Syndrome
Pathophysiology
Obstructive Sleep Apnea
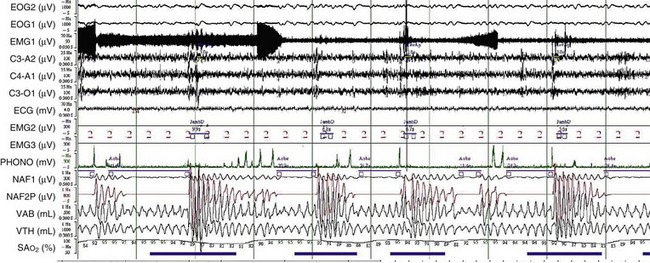
OSA may play a role in the pathogenesis of chronic hypercapnia in those patients, because in most cases, treatment of OSA corrects the hypercapnia. Indeed, to compensate for the effect of intermittent periodic breathing and resultant acute hypercapnia, normal subjects as well as patients with OSA will increase their tidal volume in the first breath after an apnea (hyperventilation). With physiologic differences in ventilatory responses between subjects, however, an overload in CO2 could result. The duration of the apneas also could contribute: When apneic episode duration becomes three times longer than the breathing interval, CO2 tends to accumulate despite maximal tidal volume, because there is insufficient time for adequate hyperventilation events (Figure 62-1). In addition, hypercapnia could blunt the ventilatory responses: The initial ventilation after an apneic episode is directly related to the volume of CO2 loaded during the preceding respiratory event and thus represents an index of CO2 ventilatory response. Hypercapnic patients demonstrated depression of this index of ventilatory compensation compared with that in eucapnic patients. It has been shown that the apnea-to-interapnea duration ratio is greater in hypercapnic patients than in eucapnic patients.
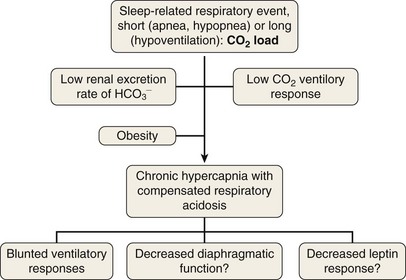
To elucidate the mechanisms that are involved in the development of hypercapnia in patients with OSA, Norman and co-workers have proposed, using a computer model, some hints for prediction of the transition from acute hypercapnia during sleep-disordered breathing (apnea, hypopnea, and hypoventilation) to chronic daytime hypercapnia. In their model, when the ventilatory CO2 response and renal HCO3− excretion were normal, increases in PaCO2 and HCO3− did not develop. The bicarbonate excretion during the day compensated for that retained during the night. When CO2 ventilatory response was very low, however, the model demonstrated a modest rise in PaCO2 and HCO3− measured during the awake state over multiple days. Similarly, when renal HCO3− excretion rate was lowered to simulate chloride deficiency, the model demonstrated a modest rise in daytime PaCO2 and HCO3−. The combination of low CO2 response and low renal HCO3− excretion rate produced a synergistic effect on the degree of elevation of daytime PaCO2. These workers suggested that hypercapnia results from an imbalance between the period of CO2 loading (short = apnea or hypopnea; long = hypoventilation) and inadequate compensation both during sleep and during the awake state. This pulmonary-renal interaction may contribute to the development and perpetuation of chronic daytime hypercapnia, which will lead to a blunted respiratory drive for the next sleep cycle (Figure 62-2).