Nonmuscular Diseases of the Chest Wall
INTRODUCTION
The chest wall is a major component of the respiratory pump and consists of the rib cage and abdomen. It is inflated by the inspiratory muscles of the rib cage and the diaphragm and its integrity is key to sustaining ventilation. Disorders affecting the nonmuscular structures of the chest wall (the thoracic spine, ribs, costovertebral joints, abdominal wall, and sternum) may lead to respiratory dysfunction. When the integrity of these nonmuscular components is severely compromised, respiratory failure may ensue. The pathophysiology of disorders affecting these nonmuscular components is generally related to the imposition of excessive elastic loads placed on the respiratory muscles. In some disorders, such as kyphoscoliosis (KS) and obesity, the load on the respiratory muscles is chronic and progressive. By contrast, with flail chest, the load on the respiratory muscles is acute. If the respiratory muscles had not adapted or had little time to adapt to loads that increase the work of breathing, respiratory failure may quickly ensue. Other disorders, such as ankylosing spondylitis (AS) and pectus excavatum, have a minimal impact on respiratory function. Diseases directly affecting the respiratory muscles are discussed in Chapter 84.
KYPHOSCOLIOSIS
Important physiologic and clinical considerations in kyphoscoliosis are discussed below.
 ETIOLOGY AND DIAGNOSIS
ETIOLOGY AND DIAGNOSIS
KS comprises a group of spinal disorders that are characterized by curvature of the spine in the lateral direction (scoliosis) and sagittal plane (kyphosis) as well as by spinal axis rotation. It is a common spinal abnormality, with estimates of prevalence in the United States ranging from 1 in 10,000 people for severe deformities to 1 in 1000 people for mild deformities.1 The etiology of KS is unknown for the vast majority of cases (85% classified as idiopathic). With the remainder, KS is due to either neuromuscular disease (paralytic or secondary KS) or is congenital (Table 83-1).2–4
Idiopathic KS usually manifests in late childhood or early adolescence and involves primarily females (ratio of 4:1).5 Individuals with idiopathic KS may complain of back pain or may have psychological problems.5 When KS is severe, complaints include dyspnea with exertion and constitutional symptoms related to nocturnal hypoventilation. Respiratory failure also may develop.6 Paralytic or secondary KS may be due to a number of neuromuscular disorders including polio, muscular dystrophy, cerebral palsy, and spinal bifida.4 With these diseases, KS typically occurs when the individuals become nonambulatory. Congenital KS results from developmental vertebral anomalies that are usually present at birth.3 Although uncommon, it can lead to serious neurological complications such as paraplegia.
The diagnosis of KS is made by physical examination and radiological assessment. In severe KS, physical examination reveals a dorsal hump involving the rib cage. This is due to angulated ribs and shoulder asymmetry. In addition, there is “hip tilt” that is related to spinal rotation (Fig. 83-1). With mild spinal deformity, kyphosis and scoliosis are less apparent when inspecting the spine and the rib cage. In this context, subtle changes in spinal curvature may be elicited by performing the Adam’s forward bend test. With this test, the patient bends forward at the waist with feet together and knees straight, until the spine becomes parallel to the floor. The observer, then, examines for thoracic or lumbar region asymmetry.
Figure 83-1 Schematic representation of the rotation of the spine and the rib cage seen with scoliosis. (Data from Bergofsky et al. Medicine (Baltimore). 1959;38:263.)
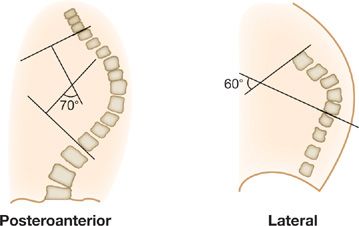
Radiographic studies (upright PA and lateral views of the spine) are used to confirm the diagnosis, and to assess severity by measuring the Cobb angle.7 This angle is formed by the intersection of two lines, each of which is parallel to the top and bottom vertebrae of the scoliotic or kyphotic curves (Fig. 83-2). A spinal deformity with a Cobb angle greater than 10 degrees is considered scoliosis.5 The greater the Cobb angle, the more severe the deformity. Cobb angles less than 60 degrees are usually not associated with ventilatory impairment, those greater than 100 degrees are invariably associated with respiratory symptoms, and those greater than 120 degrees with respiratory failure.5,8,9 Sequential radiographic studies are useful in evaluating progression of the spinal deformity.
Figure 83-2 Schematic of the posteroanterior radiograph depicting the lines constructed to measure the Cobb angle of scoliosis and the lines drawn on the lateral radiograph to measure the Cobb angle of kyphosis. (Data of Rochester, Findley, in Murray and Nadel editors, Textbook of Respiratory Medicine. Philadelphia: WB Saunders; 1988.)
 RESPIRATORY MECHANICS
RESPIRATORY MECHANICS
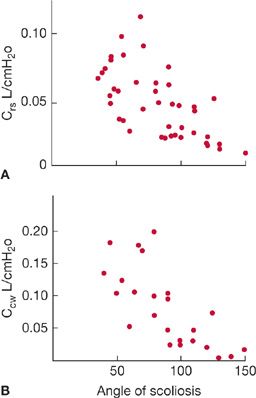
The pathophysiological hallmark of KS is reduced chest wall distensibility.10 Namely, chest wall compliance is reduced and the pressure required to inflate the chest wall at any given lung volume is greater than in a healthy individual. Consequently, functional residual capacity (FRC) is invariably decreased, the elastic load placed on the respiratory muscles is increased, and the work of breathing is significantly increased. In idiopathic KS, the compliance of the chest wall and respiratory system both decrease as the Cobb angle increases (Fig. 83-3 A,B) with the most pronounced reductions in chest wall compliance seen in individuals with Cobb angles greater than 100 degrees.10 Cobb angles up to 50 degrees do not significantly decrease respiratory system compliance.10
Figure 83-3 Relationship between (A) the angle of scoliosis and compliance of the total respiratory system and (B) the angle of scoliosis and compliance of the chest wall in patients with idiopathic scoliosis. (Reproduced with permission from Kafer ER. Mechanical properties of the respiratory system and the ventilatory response to carbon dioxide. J Clin Invest. 1975;55(6):1153–1163.)
The reduction in chest wall compliance promotes breathing with shallow tidal breaths and the reduction in FRC leads to breathing at low lung volumes. Both factors predispose to the development of atelectasis. Consequently, lung compliance may be reduced in the absence of any intrinsic lung disease. Although lung compliance may be diminished, it is not as severely affected as the chest wall. Since chest wall compliance decreases with age, respiratory mechanics invariably deteriorate with age even though the spinal deformity may not worsen over time.11
Respiratory muscle strength, as reflected in measurements of maximal static inspiratory (PImax) and expiratory pressures (PEmax), is usually normal in individuals with idiopathic KS and Cobb angles of less than 50 degrees. With Cobb angles greater than 50 degrees, there may be mild to moderate reductions in PImax and PEmax accompanied by only mild reductions in vital capacity (VC).12,13 The reductions in PImax with idiopathic KS may be due to mechanical disadvantage of the respiratory muscles coupled to a distorted rib cage.14 However, in patients with secondary KS, reductions in PImax are related to intrinsic muscle weakness.15 In these individuals, the combination of weakened inspiratory muscles and a stiff, poorly compliant, respiratory system will lead to profound decrements in VC and predispose these individuals to respiratory failure.16
The reductions in VC in idiopathic KS are typically seen in patients with severe spinal deformities (Cobb angles greater than 100 degrees). Total lung capacity (TLC) and VC may be reduced to 30% of predicted.2,8 Forced vital capacity (FVC) decreases in proportion to the reduction in TLC in the absence of obstructive airway disease. Residual volume (RV) may be normal or slightly increased. This is in contrast to the reduction in RV that occurs in restriction due to interstitial lung disease. The disproportionate reduction in TLC relative to RV in KS produces a relatively high RV/TLC ratio.2 In individuals with mild and moderate degrees of kyphosis and scoliosis (i.e., Cobb angles less than 60 degrees) VC and TLC may be only mildly reduced. Although uncommon, obstructive dysfunction in nonsmokers has been described.17 This includes reductions in midexpiratory flow rates or FEV1 resulting from tracheal displacement or torsion of major bronchi.18
Factors other than Cobb angle can contribute to the reduction in TLC and VC. These factors include the location of the spinal curve (thoracic vs. lumbar), number of vertebrae involved, patient’s age, degree of spinal rotation, and, most importantly, the presence or absence of inspiratory muscle weakness. Concomitant respiratory muscle weakness will significantly affect the severity of restriction. In patients with paralytic KS (spinal deformity secondary to neuromuscular disease), the degree of lung restriction is primarily determined by the magnitude of respiratory muscle weakness rather than by the degree of spinal curvature.15,19 Thus, for a similar Cobb angle, patients with paralytic KS are likely to have greater pulmonary function impairment than patients with idiopathic KS.15,19 In patients with congenital KS, coexisting rib deformities or underlying lung abnormalities similarly lead to a greater loss in VC for a given degree of spinal deformity than seen in patients with idiopathic KS.20
The work of breathing is increased in KS because of the elastic load related to the stiff chest wall.10 Consequently, the oxygen cost of breathing may be three to five times of that seen in healthy subjects. Concomitant inspiratory muscle weakness decreases ventilatory reserve and predisposes the respiratory muscles to fatigue and contributes to respiratory failure in these patients.16
 EXERCISE CAPACITY
EXERCISE CAPACITY
Exercise capacity may be severely limited in KS, especially when the Cobb angle is greater than 60 degrees. Cardiorespiratory dysfunction related to restrictive pulmonary function, reduced respiratory system compliance, increased work of breathing, and respiratory muscle weakness contribute to poor exercise capacity.21,22 Typically, maximal oxygen consumption is reduced to about 60% to 80% of predicted, heart rate is higher per work load, the ratio of tidal volume to vital capacity (VT/VC) is greater than 0.5, and the ratio of maximum exercise ventilation to maximum voluntary ventilation (VEmax/MVV) can reach 0.7. Concomitant pulmonary hypertension in those with Cobb angles greater than 100 degrees may independently contribute to exercise limitation.
Kyphoscoliotic individuals with mild to moderate spinal curves (Cobb angles 20–45 degrees), also may have poor exercise tolerance but any reduction in ![]() O2max more likely is due to limb muscle deconditioning rather than ventilatory constraints.23 In support of this notion, there is recent data reporting weakness of the quadriceps muscle, reduced Type I myosin, and presence of oxidative stress in the quadriceps muscle of these patients.24
O2max more likely is due to limb muscle deconditioning rather than ventilatory constraints.23 In support of this notion, there is recent data reporting weakness of the quadriceps muscle, reduced Type I myosin, and presence of oxidative stress in the quadriceps muscle of these patients.24
 CONTROL OF BREATHING
CONTROL OF BREATHING
Advanced KS is associated with significant alterations in breathing pattern. Typically, individuals with severe KS adopt a rapid shallow breathing pattern consisting of low tidal volumes, shortened inspiratory time (TI), and an increased respiratory rate (i.e., reduced total breath time, TTOT).16,25 In young patients with KS and normal blood gases, TI and duty cycle (TI/TTOT) correlate negatively with the angle of scoliosis.25 These changes in breathing pattern likely represent an adaptation to chest wall restriction. The benefit of using this breathing pattern is twofold. First, the work per breath is decreased. Second, the ratio of the pressure needed to inhale (Pbreath) to PImax is reduced. In theory, reducing Pbreath/PImax lessens the likelihood of developing inspiratory muscle fatigue and respiratory failure. The disadvantage of a rapid shallow breathing pattern is that it promotes atelectasis, causes hypoxemia, and may lead to a further reduction of lung compliance.
A second means of adapting to the added elastic load of the stiffened chest wall is to increase respiratory drive.25 Indirect measurements of the neural drive to the respiratory muscles, such as mouth occlusion pressure at 100 ms (P0.1) are usually elevated in these individuals and correlate positively with the degree of scoliosis.25 The increase in neural drive is not necessarily associated with increased alveolar ventilation as the stiff chest wall and the weak respiratory muscles will limit any augmentation in alveolar ventilation.
 SLEEP DISORDERED BREATHING
SLEEP DISORDERED BREATHING
Nocturnal hypoventilation may occur in severe KS and typically predates the development of chronic hypercapnia.9,26 While awake, individuals with Cobb angles greater than 100 degrees can maintain adequate alveolar ventilation by increasing respiratory drive to the diaphragm and recruiting accessory inspiratory muscles.16,25 However, during sleep, neural drive to all the inspiratory muscles is diminished, especially during REM sleep.27 Since these patients are dependent exclusively on the diaphragm to maintain alveolar ventilation in REM sleep, they are at risk for becoming hypercapnic and hypoxemic. The risk of hypoventilation is amplified if there is any underlying diaphragm dysfunction. Of interest, the magnitude of nocturnal hypoventilation may not correlate with the degree of thoracic deformity.28 This observation suggests that inspiratory muscle weakness may be a more important factor for developing nocturnal hypoventilation. The presence of sustained nocturnal desaturation and hypercapnia may further weaken the respiratory muscles, compound the hypoxemia, lead to pulmonary hypertension and eventually cor pulmonale. Obstructive sleep apnea, which has a similar prevalence in KS to that of the general population, also may complicate nocturnal hypoventilation.29 Since nocturnal hypoventilation occurs before development of the typical symptoms and signs of cardiorespiratory failure, the clinician must be alert to sleep-related breathing abnormalities in these patients and promptly diagnose them with overnight polysomnography.29
 GAS EXCHANGE
GAS EXCHANGE
Abnormalities in gas exchange are frequently found in patients with KS; normocapnic hypoxemia being the most common abnormality. In nonsmokers with idiopathic KS, PaO2 correlates directly with VC and inversely with the angle of scoliosis. The age-dependent decrease in PaO2 is greater than that of normal individuals and oxyhemoglobin desaturation can occur with minimal activity.11,30 Hypoxemia is primarily due to ventilation–perfusion mismatch and less often to intrapulmonary shunt associated with atelectasis.30 In severe KS with chronic hypercapnia, hypoventilation also contributes to hypoxemia. Hypercapnia is initially detected only during sleep or with exercise.26 With aging or as the disease progresses, hypercapnia is seen at rest while awake.
 CLINICAL COURSE
CLINICAL COURSE
Individuals with congenital KS and severe spinal deformities may exhibit a rapidly deteriorating clinical course resulting in pronounced restrictive dysfunction, cor pulmonale, and early death.6,31 Those with secondary KS associated with neuromuscular disease may similarly progress to develop severe restrictive dysfunction and cardiorespiratory failure.32 In general, when KS manifests early in life (age 0–8 years) there is a greater risk for rapid progression of the spinal deformity during growth, continued progression of the deformity after skeletal maturity, and a greater likelihood of developing respiratory failure.31
Individuals with idiopathic KS typically have a more benign course. If the spinal deformity at skeletal maturity is mild, they have an excellent prognosis with little respiratory impairment, and an overall good quality of life.5 In individuals with idiopathic KS and moderate or severe deformity at presentation (age 12–16 years), the risk of deformity progression and development of respiratory impairment increases significantly.5 Progressive curvature of the spinal deformity is, in general, greater in skeletally immature individuals, in those with large deformities at presentation, and in those whose curves have a thoracic apex.5 By rough estimates, individuals with thoracic deformities greater than 50 degrees at skeletal maturity are at risk for a progressive increase in the spinal curve at a rate of about 1 degree annually.33
Most young individuals with idiopathic KS are asymptomatic. As they age, many will develop back pain and dyspnea. Initially dyspnea may only be problematic with activities and eventually may be present at rest if the spinal deformity progresses.11 Consequently, individuals with severe idiopathic KS, especially those with a Cobb angle greater than 110 degrees and VC of less than 45% of predicted, should be monitored for respiratory compromise as they age.11 Once cardiorespiratory failure develops, the prognosis is poor; without supplemental oxygen or ventilatory support death may occur within 1 year. The risk of respiratory failure is even greater in patients with inspiratory muscle weakness, sleep disordered breathing, obesity, or concomitant obstructive airway disease. Pregnancy poses no added risk for respiratory failure; however, women with idiopathic KS and a VC reduced to less than 1 L may develop respiratory problems during pregnancy.
 TREATMENT
TREATMENT
General supportive measures for adults with KS include smoking cessation, maintenance of body weight within a desirable range, engaging in frequent physical activity to improve exercise capacity and minimize deconditioning, immunization against influenza and pneumococci, prompt treatment of respiratory infections, and psychological support for those with diminished self-esteem. Supplemental oxygen may be needed with activity or during sleep if there is evidence of oxyhemoglobin desaturation with exertion or with sleep. Treatment with supplemental oxygen alone may be sufficient if it corrects nocturnal hypoxemia without inducing hypercapnia.34 However, the development of hypercapnia needs to be closely monitored, especially in individuals with a VC of less than 50% of predicted. If hypercapnia is present, nocturnal noninvasive ventilatory support should be initiated. Dyspnea, morning headaches, poor sleep quality, fatigue, and the presence of cor pulmonale are symptoms and signs that should alert the clinician to the presence of nocturnal hypoventilation.35 Long-term, nocturnal noninvasive ventilation should be offered to all patients with KS and chronic hypercapnic respiratory failure as it reduces mortality (Canadian Thoracic Society, grade 1B).34,36
Noninvasive positive pressure ventilation can be delivered via a nasal mask or full-face mask, with or without supplemental oxygen. When prescribing a positive pressure device, either a pressure- or volume-preset ventilator can be used.9 Apart from the potential for greater leakage with the pressure mode, pressure or volume ventilation has equivalent beneficial effects on physiological and clinical parameters as well as overall health status. Contraindications to noninvasive ventilation include the inability to protect the upper airway due to impaired cough, excessive airway secretions, or inability to cooperate.35 When noninvasive ventilation has failed or is contraindicated, invasive ventilation should be considered.
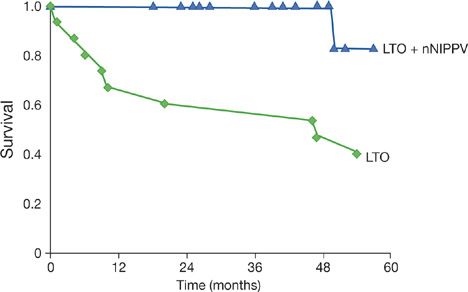
Benefits of noninvasive ventilation include improvements in quality of life, gas exchange, sleep efficiency, pulmonary hemodynamics, respiratory drive, and survival (Table 83-2).9,37 By contrast, measurements of VC, PImax, twitch transdiaphragmatic pressure and respiratory muscle endurance are not improved by noninvasive ventilation.38 Accordingly, the lower incidence of respiratory failure in patients undergoing positive pressure ventilation may be attributed to improvements in respiratory control rather than improvement of respiratory muscle contractility.38 Long-term noninvasive ventilation considerably reduces the number of hospitalizations for respiratory failure and the length of hospital stay.9 Survival benefit of KS patients treated with noninvasive ventilation and supplemental oxygen over those patients treated with just oxygen has been demonstrated by comparative and observational studies (Fig. 83-4).36
TABLE 83-2 Therapeutic Benefits of Noninvasive Mechanical Ventilation in Patients with Kyphoscoliosis
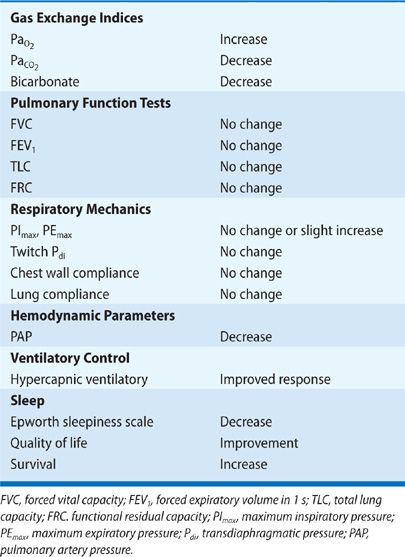
Figure 83-4 Survival curves of kyphoscoliotic patients treated with long-term oxygen therapy (LTO) and nocturnal nasal intermittent positive pressure ventilation (nNIPPV). (Reproduced with permission from Buyse B, Meersseman W, Demedts M. Treatment of chronic respiratory failure in kyphoscoliosis: oxygen or ventilation? Eur Respir J. 2003;22(3):525–528.)
Traditional surgical approaches to correct spinal deformities in skeletally immature children have included spinal fusion and/or implantation of rods. Spinal fusion has considerable disadvantages as it has been associated with restrictive respiratory defects, decreased ability to carry out daily activities, and poor cosmetic results.39 In young children (age <10 years), severe restrictive respiratory disease (VC <50% of predicted) may occur in roughly 50% of patients undergoing spinal fusion, especially in those with extensive thoracic fusions or in those with fusions of the proximal thoracic spine.39 Surgical fusion of the spine may have a role in children with congenital spinal deformities or in children with KS secondary to neurological defects. A nonfusion approach includes implantation of growth-friendly rods such as expandable spinal rods, titanium rib implants, or remotely distractible magnetically controlled rods.40 These newer techniques permit control of spinal deformity while allowing continued spinal growth and pulmonary development.40
THORACOPLASTY
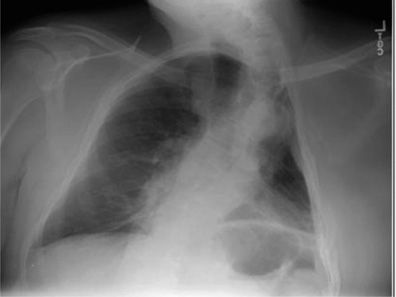
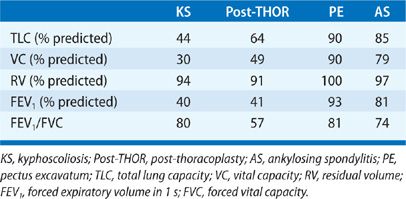
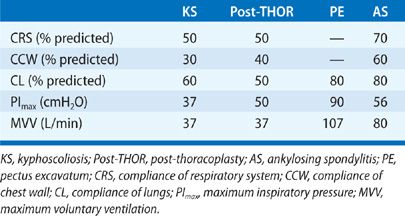
In the pre-antituberculous chemotherapy era, thoracoplasty was the standard surgical approach to control pulmonary tuberculosis. The surgery consisted of different combinations of rib removal, rib fracture, phrenic nerve resection, or lung compression by filling the pleural space with foreign material (i.e., ping pong balls) (Fig. 83-5). Since the procedure was primarily performed in the 1940s and 1950s, very few people who had undergone the procedure are still alive.41,42 Historically, these individuals commonly developed dyspnea, severe restrictive dysfunction, and chronic hypercapnic respiratory failure as they aged.41,42 The severity of the restrictive defect was often comparable to that seen in KS (Tables 83-3 and 83-4) and was related to the number of ribs removed, presence of fibrothorax, lung fibrosis secondary to underlying granulomatous disease, prior lung resection, or intentional phrenic nerve damage.41,42 As in most restrictive chest wall diseases, these patients had impaired gas exchange, limited exercise tolerance, an increased oxygen cost of breathing, and eventually would develop cor pulmonale. Progressive scoliosis with aging further impaired respiratory function. Although this procedure is of historical interest, the purpose of reviewing thoracoplasty in this chapter is that the procedure is still occasionally performed. Currently, thoracoplasty is indicated for treatment of bronchopleural fistulae that have failed to close following decortication or for treatment of persistent empyema in which decortication is not feasible or has failed to eradicate the infection.43 Treatment of individuals who are postthoracoplasty and have developed restrictive disease consists of supplemental oxygen, antibiotics for respiratory infections, and noninvasive ventilatory support.
Figure 83-5 Chest radiograph of a patient with a history of Mycobacterium tuberculosis, demonstrating marked deformity of the left hemithorax consistent with prior thoracoplasty.
PECTUS EXCAVATUM
Clinical considerations in pectus excavatum are discussed below.
 ETIOLOGY AND CLINICAL FEATURES
ETIOLOGY AND CLINICAL FEATURES
Pectus excavatum, also known as funnel chest, refers to a congenital chest wall deformity characterized by excessive depression of the sternum and the adjacent ribs producing a caved-in appearance to the rib cage.44 It is the most common congenital deformity of the chest wall, with a prevalence of about 1 in 1000 live births and a male to female ratio of 3 to 1.45 A family history may be present in 15% to 40% of cases.46 It is believed to result from an abnormal growth of cartilage around the sternum due to a defect in collagen formation.44 In support of this mechanism, is the higher prevalence of pectus excavatum in Marfan syndrome.47 However, the pathogenesis of pectus excavatum in the majority of cases is unknown.
The sternal depression may be minimal or prominent. In extreme cases, it is usually apparent at birth and progresses as the child grows. The most frequent complaints of individuals with pectus excavatum are cosmetic. The psychological impact of the pectus deformity becomes more troublesome during the teenage years when body image becomes more important. Exertional dyspnea and exercise intolerance may occur in 30% to 70% of patients.44 However, these symptoms cannot be entirely explained by the mild restrictive pattern, which is sometimes seen (Table 83-3) or by limitations in cardiac output.
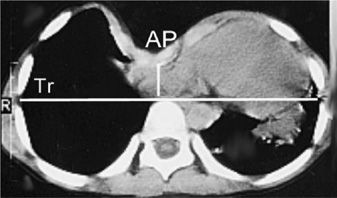
The degree of sternal depression is best assessed with a chest CT scan. The ratio of the transverse to anterior–posterior (AP) diameters of the rib cage at the level of the deepest sternal depression (Haller index) can easily be measured using this technique (Fig. 83-6).44 In normal individuals this ratio is about 2.5. A ratio greater than 3.25 is considered significant sternal depression.44
Figure 83-6 Chest computed tomography of a patient with pectus excavatum. The distance between the anterior aspect of the vertebral body and the posterior aspect of the sternum (AP) is decreased. The Haller index is calculated as the ratio of the internal transverse diameter (Tr) to AP distance.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Severe pectus deformities may be associated with mild restriction on pulmonary function tests. A Haller index of 5 or higher can be associated with mild reductions in TLC and VC. The restriction may be more pronounced if there is concomitant scoliosis.48 Although obstructive defects are infrequent (approximately 2% of cases)48 RV and the RV/TLC ratio are commonly increased. The elevated RV and RV/TLC may be due to stiffness of the rib cage resulting in reduced chest wall compliance in these young individuals rather than air trapping or expiratory muscle weakness. In most cases there is no underlying lung disease, and lung compliance is normal. Unlike AS, the mobility of the rib cage is not impaired at rest or during exercise in pectus excavatum.49,50
Cardiopulmonary exercise testing is usually normal in individuals with pectus excavatum. Indices such as maximal work rate, maximum oxygen consumption, and maximal oxygen pulse are not different from those of healthy individuals. In severe deformities, limited exercise capacity with reductions in maximal work rate or oxygen consumption at a given work rate may occur and often are out of proportion to the mild restrictive respiratory impairment found in these patients.51 The explanation for the limited exercise capacity in this group of patients may be a reduction in venous return to heart associated with right ventricular compression due to sternal depression. Distortion of right ventricular geometry may be detected by echocardiographic or cardiac magnetic resonance imaging studies.52 However, the role of these findings in limiting exercise capacity is not entirely known.
 TREATMENT
TREATMENT
Although surgery is most often performed for cosmetic reasons, it is clearly indicated when the deformity has significant physiological and psychological repercussions for the individual.45,53 The optimal timing for repair is around 10 to 14 years of age or at a younger age if there is significant cardiopulmonary compromise. Individuals selected for repair typically have a Haller index greater than 3.25.45,53
The most common surgical approaches include modifications of the open-resection method originally described by Ravitch or the minimally invasive approach described by Nuss.44 The first method involves resection of costal cartilage and a sternal osteotomy, with or without fixation of the sternum by external or internal supports. This procedure may be complicated by sternal necrosis, infection, or recurrence of the deformity. Recurrence may arise in younger children in whom sternal supports are not used. The invasive approach is best suited for individuals with combined pectus excavatum and carinatum, marked asymmetry, or extensive defects involving the upper ribs.44
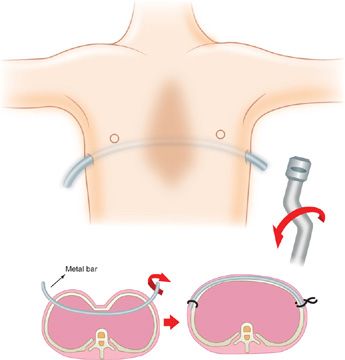
The Nuss procedure, developed in the 1990s, provides a minimally invasive alternative to reconstructing the deformity.54 It consists of placing a curved metal bar under the sternum at the point of deepest depression through small incisions made on each side of the rib cage. The sternum is then pushed forward and stabilized by the metal bar (Fig. 83-7). With this procedure, the costal cartilage is not resected. The bar is generally left in place for 2 to 4 years, resulting in permanent chest wall remodeling. Complications of this approach include bar displacement or rotation that would require reoperation, as well as pneumothorax, pericarditis, and infection. The use of thoracoscopic techniques has minimized associated complications. Overall, the rate of complications does not differ significantly between the two surgical approaches.55 Deformity repair with either procedure impacts positively on the psychosocial well-being of the individual.55
Figure 83-7 Schematic depicting the Nuss procedure in a patient with pectus excavatum. A curved bar is inserted behind the sternum and then rotated to displace the depressed sternum ventrally.
Improvements in cardiopulmonary function with invasive or minimally invasive procedures remain controversial.56 If there is improvement, it is mostly modest and clinically insignificant. Improvements in exercise tolerance, cardiac output, and ![]() O2max after surgical correction have been described in some but not all studies.56,57 Discrepancies among studies may be due to differences in patient populations (younger vs. older patients), surgical techniques, the interval at which tests are performed following the operation, or the effects of growth on pulmonary function. One year following surgery, any improvement in pulmonary function is independent of which surgical procedure was performed.
O2max after surgical correction have been described in some but not all studies.56,57 Discrepancies among studies may be due to differences in patient populations (younger vs. older patients), surgical techniques, the interval at which tests are performed following the operation, or the effects of growth on pulmonary function. One year following surgery, any improvement in pulmonary function is independent of which surgical procedure was performed.
ANKYLOSING SPONDYLITIS
Important physiologic and clinical considerations in ankylosing spondylitis are described below.
 ETIOLOGY AND CLINICAL FEATURES
ETIOLOGY AND CLINICAL FEATURES
AS is a chronic multisystem disease characterized by inflammation of the spine, sacroiliac and peripheral joints as well as involvement of extra-articular organs including the lungs and the heart.58 Chronic inflammation of the spinal structures, costovertebral, apophyseal, and sacroiliac joints leads to fibrosis and ossification of these structures.59 Consequently, spinal mobility is reduced and there is considerable limitation of rib cage expansion.60
AS affects approximately 0.1% of the general population and is more common in men than in women, with a ratio of 16:1. Its etiology is unclear. There is a genetic predisposition for AS, as 95% of whites with AS have the HLA-B27 antigen.58 Clinically, patients in late adolescence or early adulthood, present with back pain or morning stiffness due to involvement of sacroiliac joints. Onset of symptoms after the age of 45 is rare. Typically, the symptoms are worse in the morning or after prolonged rest. About 30% of individuals with AS have involvement of a peripheral joint or nongranulomatous anterior uveitis. Aortitis and dilatation of the aortic root may occur in up to 25% of patients.
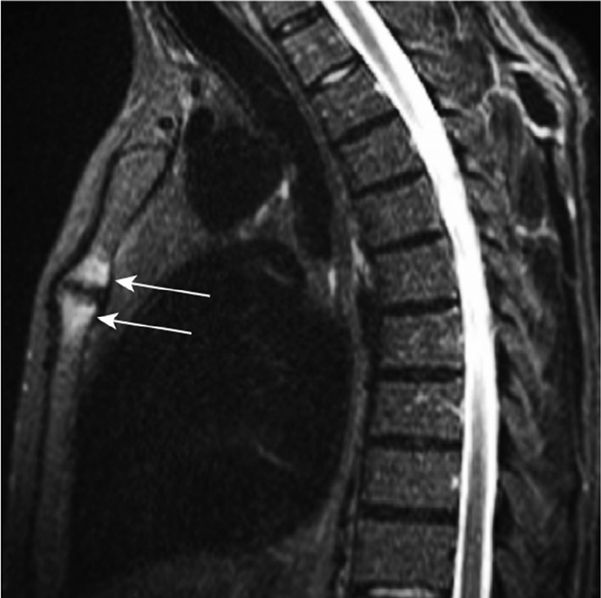
Chest pain due to inflammation of manubriosternal junction and/or the sternoclavicular joints with inability to fully expand the chest on inspiration can occasionally occur (Fig. 83-8).61 On physical examination, there may be tenderness of the anterior chest wall, the costochondral region, or the manubriosternal junction. Chronic back pain may cause sleep fragmentation resulting in difficulties with daytime somnolence and fatigue.62 Upper airway obstruction due to cricoarytenoid cartilage involvement is a rare complication.63
Figure 83-8 Inflammatory lesions of the anterior chest wall displayed by whole-body MRI in an ankylosing spondylitis patient complaining of anterior chest wall pain. Arrows point to bone marrow edema in both parts of the manubriosternal joint. (Modified with permission from Weber U, Lambert RGW, Rufibach K et al. Anterior chest wall inflammation by whole-body magnetic resonance imaging in patients with spondyloarthritis: lack of association between clinical and imaging findings in a cross-sectional study. Arthritis Research & Therapy. 2012;14(1):R3.)
Individuals with advanced AS have very limited rib cage motion and are more dependent on diaphragmatic breathing. Prominent abdominal excursion during inspiration may be apparent in supine or upright individuals. The degree of rib cage immobility can be assessed by measuring the change in rib cage circumference at the level of the fourth intercostal space between a full inspiration and full expiration. Rib cage expansion less than 2.5 cm should raise the possibility of AS in young patients with chronic low back pain.
 RESPIRATORY PATHOPHYSIOLOGY
RESPIRATORY PATHOPHYSIOLOGY
Limited rib cage expansion is the pathophysiological hallmark of AS.59,64,65 This limitation results from fusion of the costovertebral and sternoclavicular joints and possibly leads to intercostal muscle atrophy.59 Rib cage motion is similar to that in healthy individuals in terms of direction, but the extent of movement is diminished.60 Rib cage immobility leads to a reduction in chest wall and total respiratory system compliance but lung compliance is generally normal (Table 83-4).65
Only mild reductions in VC or TLC (75%–80% of predicted) may be present despite moderately severe reductions in rib cage mobility (Table 83-3).66 The restrictive impairment is associated with disease activity, disease duration, and spinal mobility.66,67 Mild reductions of respiratory muscle strength (PImax, and PEmax) may be recorded in individuals with severely limited rib cage expansion. However, diaphragmatic strength is typically intact in these patients.59 Since rib cage expansion is severely limited in advanced AS, chest wall inflation is primarily accomplished through diaphragmatic displacement of the abdomen (Fig. 83-9). This pathway accounts for most of the change in tidal volume during quiet breathing, speech, or exercise.68 In this setting, the increased diaphragm shortening and the relatively greater transdiaphragmatic pressure required to inflate a stiff rib cage59 may potentially provide a training stimulus to the diaphragm.
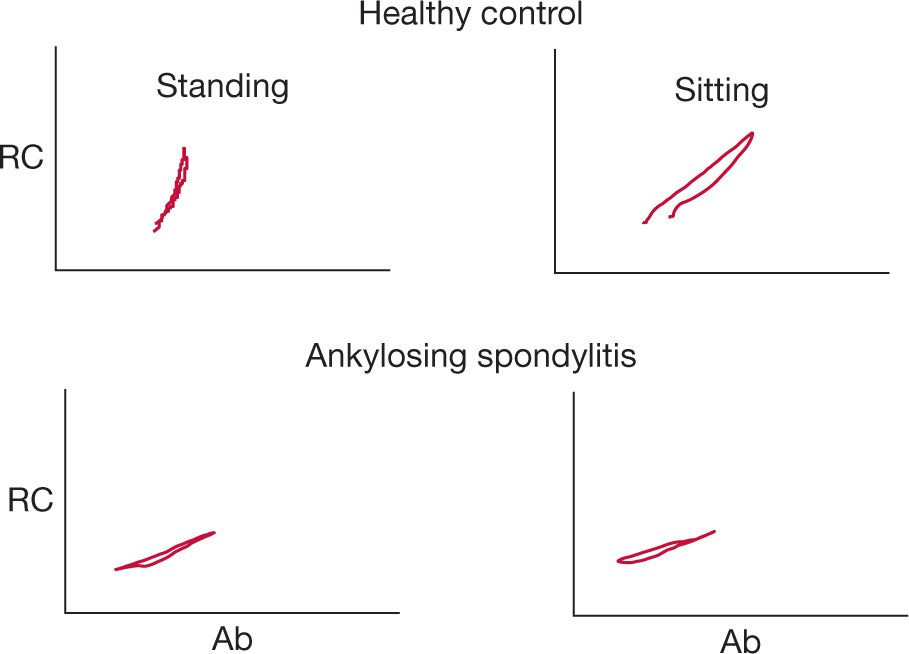
Figure 83-9 Changes in anteroposterior dimensions of the rib cage (RC) and abdomen (Ab) in a healthy individual and one with ankylosing spondylitis (AS). There is limited mobility of the rib cage in all positions resulting in greater motion of the abdomen relative to the rib cage.
In the absence of parenchymal lung disease, PaO2 is either in the normal range or mildly reduced.66 Exercise capacity may be mildly decreased in patients with AS, especially in those with marked rib cage restriction.69 The mechanism of limited exercise is most likely related to peripheral deconditioning rather than ventilatory constraints.69
 PLEUROPULMONARY ABNORMALITIES
PLEUROPULMONARY ABNORMALITIES
Upper lobe fibrobullous disease occurs in a small percentage (up to 4%) of individuals with AS.70,71 It is more common in males with long-standing disease and may manifest as interstitial infiltrates, fibrosis with honeycombing, or cavitation that may mimic tuberculosis.70,71 The pathogenesis of upper lobe fibrobullous disease is unknown. Possible mechanisms include decreased upper lobe ventilation, mechanical stress due to rib cage rigidity, and recurrent lung infection due to impaired cough. Individuals with fibrobullous disease have an increased propensity for spontaneous pneumothorax and infections with Aspergillus or atypical mycobacteria.71 Fibrobullous disease tends to be progressive and its course is not altered by steroid treatment. Because resection of the lung segment with fibrobullous disease can be complicated by bronchopleural fistula in 50% to 60% of patients, surgery is reserved for treatment of massive hemoptysis.71 Additional pleuropulmonary abnormalities that can be detected by chest CT include interstitial lung disease, pleural thickening, parenchymal bands, and bronchial wall thickening.70 These abnormalities are subtle and do not correlate with clinical or functional impairment.
 SLEEP DISORDERED BREATHING
SLEEP DISORDERED BREATHING
An increased prevalence of obstructive sleep apnea in AS has been reported, especially in patients with long-standing disease (>5 years).72,73 Because fatigue is a common complaint in AS, clinicians should have a high index of suspicion for concomitant sleep disordered breathing contributing to excessive daytime sleepiness and screen these patients with polysomnography.72,73
 TREATMENT
TREATMENT
Treatment of individuals with AS should incorporate pain relief, physical therapy, and measures to maintain posture. Physical therapy exercises that promote rib cage expansion have beneficial effects on pain and spinal mobility. Smoking should be avoided and baseline chest radiographs and spirometry obtained.
Over the past decade, the use of antagonists of tumor necrosis factor (TNF) has had a dramatic effect on pain control, fatigue, spinal flexibility, and quality of life in these patients.74 Rib cage expansion is also improved following treatment with biological agents. The effectiveness of TNF-blockade can be maintained for several years with continued treatment. Since anti-TNF agents do not prevent new bone formation in AS patients, their overall effects on the natural history of the disease await further assessment.75 An adverse effect of the anti-TNF therapy is reactivation of tuberculosis. Therefore, AS patients who are candidates for anti-TNF therapy should be screened for latent tuberculosis and receive prophylactic treatment with isoniazid before starting treatment.76 Additional rare adverse pulmonary complications of anti-TNF blockade include interstitial lung disease and development of a sarcoid-like disorder.76
OBESITY
Obesity is a prevalent problem. Important clinical considerations are discussed below.
 CLINICAL FEATURES
CLINICAL FEATURES
Obesity has reached an alarming prevalence throughout the world. It is estimated that about two-thirds of adults in the United States are either overweight or obese and 20% of children are obese.77 The World Health Organization has declared obesity one of the top ten risk conditions worldwide and one of the top five in developed countries.78 Obesity is associated directly or indirectly with several comorbidities, increased disability and decreased life expectancy.79 It is a significant contributor to escalating healthcare costs.
Severity of obesity is commonly assessed by calculating the body mass index (BMI), defined as body weight (BW) in kilograms divided by the square of height (Ht) in meters (BW/Ht2).80 An individual with a BMI between 18.5 and 24.9 kg/m2 is normal; a BMI between 25 and 29.9 kg/m2 is overweight, and a BMI greater than 30 kg/m2 is considered obese; BMI greater than 40 kg/m2 is considered morbid obesity. Morbidity and premature mortality usually begin to increase at a BMI of 25 to 29.9 kg/m2 and further increase with a BMI greater than 30 kg/m2.
Obesity-associated respiratory morbidity results primarily from derangements in chest wall mechanics and the control of breathing.81,82 Obesity may decrease lung volumes, decrease respiratory muscle strength, increase the work of breathing and reduce respiratory drive.81,82 Despite these limitations, most morbidly obese patients remain eucapnic; referred to as simple obesity (SO). However, a subgroup of morbidly obese individuals develops hypercapnia.81 These individuals have obesity hypoventilation syndrome (OHS) (Table 83-5).