Noninvasive Assessment of Peripheral Vascular Disease
Michael R. Jaff
Steven Deitelzweig
Overview
With the rapid proliferation of effective, safe, and minimally invasive alternatives to treat patients with a myriad of noncoronary vascular disorders, it is no surprise that interest in understanding the intricacies of vascular testing has risen to previously unmatched levels among cardiovascular specialists. As many interventional cardiologists gain experience in the endovascular treatment of peripheral vascular disease (PVD), the need to develop testing algorithms for the diagnosis and surveillance of these disorders is critical. In addition, the Core Curriculum in Cardiology for Adult Cardiovascular Training Programs (COCATS-2) recently described mandatory training for all fellow candidates in adult cardiovascular training programs in vascular medicine, including knowledge of vascular diagnostic testing (1). This chapter is designed to provide a broad overview of the available tests, including advantages, limitations, and alternatives. Obviously, there is insufficient space to provide a level of detail that would allow for the development of expertise solely by reviewing this chapter. However, the information provided will allow for an assessment of areas of interest and need for further study.
History and Physical Examination in the Diagnosis of Peripheral Vascular Disease
Contrary to many other disorders in which diagnostic testing is often the most important component in the evaluation of patients, the history and physical examination is critical. Peripheral arterial disease (PAD) is the optimal example. Without a thorough understanding of the symptoms and physical findings, diagnostic testing will only provide an anatomic assessment, but will not assist in determining optimal therapy. If a patient has minimal symptoms of intermittent claudication which does not particularly limit physical functioning, the finding of a 30-cm occlusion of the superficial femoral artery is superfluous.
In addition, cardiovascular specialists, already possessing skills in the physical examination of the heart, must gain expertise in the peripheral vascular examination. Auscultation for bruits over the cervical, abdominal, flank, and femoral regions must be routinely performed. Palpation of all pulses, including superficial temporal (a diminished superficial temporal pulse may suggest an important carotid bifurcation stenosis), carotid, subclavian, axillary, brachial, radial, ulnar, femoral, popliteal, dorsalis pedis, and posterior tibial pulses, is important to perform and record. Grading of pulse intensity is commonly noted as follows: absent (grade 0); diminished (grade 1); and normal (grade 2). An aneurysm of the popliteal artery should be noted as such and not as grade 2 + or grade 4. Examination of the feet and toes must become a routine component of the physical examination. Subtle findings of tinea pedis in the intertriginous regions of the toes may result in ischemic ulcerations in patients with PAD and diabetes mellitus, for example (Fig. 106.1).
Vascular Diagnostic Laboratory: Basic Components
Once the suspicion for PVD has been raised, the initial objective diagnostic testing commonly begins in the vascular diagnostic
laboratory. Although ranging in size and scope of services, a comprehensive vascular diagnostic laboratory provides objective testing for physiologic evaluation of lower and upper extremity arterial disease, as well as of cerebrovascular, aortic, renal mesenteric, and venous diseases. Current commercially available equipment includes limb blood pressure cuff devices continuous wave hand-held Doppler units, color duplex ultrasonography (DUS) units, and, more recently, portable DUS devices.
laboratory. Although ranging in size and scope of services, a comprehensive vascular diagnostic laboratory provides objective testing for physiologic evaluation of lower and upper extremity arterial disease, as well as of cerebrovascular, aortic, renal mesenteric, and venous diseases. Current commercially available equipment includes limb blood pressure cuff devices continuous wave hand-held Doppler units, color duplex ultrasonography (DUS) units, and, more recently, portable DUS devices.
 FIGURE 106.1. Patient with diabetes mellitus, dense peripheral sensory neuropathy, and advanced peripheral arterial disease with critical limb ischemia. |
Tests are performed by trained vascular technologists, preferably possessing the registered vascular technologist (RVT) certification demonstrating a minimum level of training, expertise, and experience. Physicians who interpret these studies commonly receive formal training during fellowship, or they must receive training in an accredited vascular diagnostic laboratory, proctored by physicians with appropriate credentials. Images are commonly stored on Picture Archiving Computerized Systems in digital format that provide the highest-quality images for evaluation and interpretation.
Accreditation of Vascular Diagnostic Laboratories
Vascular diagnostic laboratories may be found in large tertiary urban medical centers, rural community hospitals, multispecialty physician outpatient facilities, and single physician offices. Despite the variability in location and scope of services, certain components of organization, credentialing, testing algorithms, quality assurance, accuracy, and continuing education must be constant from laboratory to laboratory. Although compliance is still voluntary, many vascular laboratories around the United States submit comprehensive applications for credentialing by either the American College of Radiology (http://www.acr.org/s_acr/sec.asp?CID=594&DID=14258) or the Intersocietal Commission for the Accreditation of Vascular Laboratories (ICAVL) (http://www.icavl.org). The ICAVL is sponsored by multiple professional vascular organizations and provides a rigorous application process to ensure the public and physicians of a minimum degree of accuracy and quality. There is a trend among many state legislators to require accreditation for full reimbursement of vascular laboratory tests.
Testing in Peripheral Arterial Disease
Physiologic Testing
Ankle-Brachial Index, Segmental Limb Pressures, Pulse Volume Recordings, and Exercise Testing
The ankle-brachial index (ABI) is the easiest, most widely reproducible, and most accurate method of determining the degree of diminished arterial circulation in a limb. Using a standard sphygmomanometer and a hand-held continuous wave Doppler probe, the higher of the two pedal pressures (dorsalis pedis or posterior tibial artery) is compared with the higher of the two brachial blood pressures, and a ratio is obtained. Given that the systolic pressure normally increases in the peripheral arteries (predominantly owing to increased resistance of these vessels), a normal ABI is 0.9 or greater. An ABI less than 0.5 suggests significant arterial disease and likely represents multisegmental involvement (Table 106.1). Although there is variability in sequential ABI measurements (2), the ABI can objectively demonstrate improvement in lower limb circulation at rest.
In addition to the utility of the ABI in determining the presence of PAD, the ABI has also been used to identify the prevalence of PAD in populations at risk (3), and it defines risk for major adverse cardiovascular events (myocardial infarction, stroke) and cardiovascular death (4).
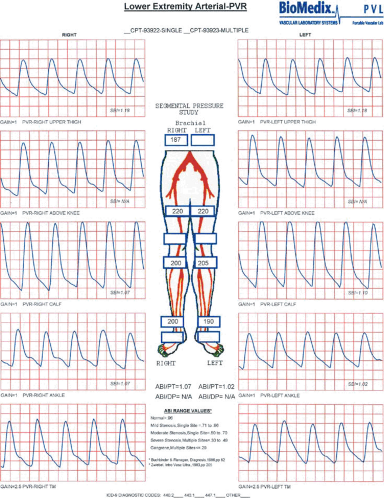
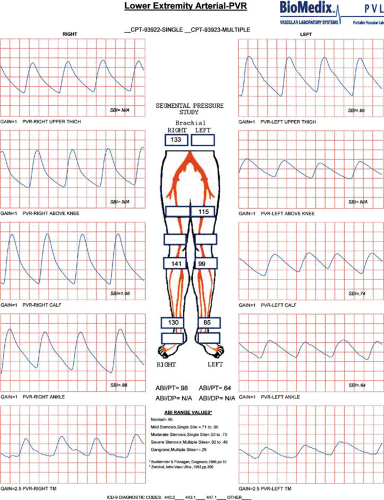
The addition of segmental limb pressures can aid in localizing segments of disease involvement. A series of limb pressure cuffs is placed on the thigh (some centers prefer high- and low-thigh cuffs), calf, ankle, transmetatarsal region of the foot, and digit. The ABI is calculated, and then the pressure is sequentially inflated in each cuff to approximately 20 to 30 mm Hg above systolic pressure. Utilizing a continuous wave Doppler probe placed at a pedal vessel, the pressure in the cuff is gradually released, and the pressure at each segment is measured. If a decrease in pressure between two consecutive levels greater than 30 mm Hg is identified, this suggests arterial disease of the artery proximal to the cuff. In addition, comparing the two limbs, a 20- to 30-mm Hg discrepancy from one limb to the other at the same cuff level also suggests significant arterial stenosis or occlusion proximal to the cuff (5) (Fig. 106.2).
The ABI can be a highly accurate method of determining the presence of PAD and its severity. However, if the ankle vessel is calcified, as is commonly seen in patients with diabetes mellitus or end-stage renal disease, an accurate ankle pressure cannot be obtained. The pressure in these calcified arteries is often greater than 200 mm Hg. If this result is not recognized as artifactually high, the physician may falsely conclude that arterial circulation is adequate or even normal, in these patients. In
this situation, other tests are available in the vascular diagnostic laboratory, including photoplethysmography, digital pressures, arterial DUS, and even assessments of wound healing potential utilizing transcutaneous oximetry. Digital pressures are most commonly utilized in this situation (6). Absolute measurements of digital pressures (using photoplethysmography) lower than 30 mm Hg in association with diminished pulse wave amplitudes in the digit suggest inadequate arterial circulation to promote wound healing (7).
this situation, other tests are available in the vascular diagnostic laboratory, including photoplethysmography, digital pressures, arterial DUS, and even assessments of wound healing potential utilizing transcutaneous oximetry. Digital pressures are most commonly utilized in this situation (6). Absolute measurements of digital pressures (using photoplethysmography) lower than 30 mm Hg in association with diminished pulse wave amplitudes in the digit suggest inadequate arterial circulation to promote wound healing (7).
TABLE 106.1 Ankle-Brachial Index and Peripheral Arterial Disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Pulse volume recordings (PVRs) are plethysmographic tracings that detect the changes in the volume of blood flowing through a limb. Using similar equipment as described previously, the cuffs are inflated to 65 mm Hg, and a plethysmographic tracing is recorded at various levels (8). The normal
PVR is similar to the normal arterial pulse wave tracing and consists of a rapid systolic upstroke and rapid downstroke, with a prominent dicrotic notch. With increasing severity of disease, the waveform becomes more attenuated, with a wide downslope and, ultimately, virtually absent waveforms. An example of normal right leg PVR and segmental pressure tracings and abnormal left leg tracings suggestive of left superficial femoral artery disease is shown (Fig. 106.3).
PVR is similar to the normal arterial pulse wave tracing and consists of a rapid systolic upstroke and rapid downstroke, with a prominent dicrotic notch. With increasing severity of disease, the waveform becomes more attenuated, with a wide downslope and, ultimately, virtually absent waveforms. An example of normal right leg PVR and segmental pressure tracings and abnormal left leg tracings suggestive of left superficial femoral artery disease is shown (Fig. 106.3).
ABIs, segmental pressures, and PVRs are useful objective tests in patients with suspected PAD, in those with limb
discomfort without an obvious cause, as a method of evaluating the success of an intervention, and as a method of follow-up. The test is inexpensive, painless, reproducible, and relatively easy to perform. The equipment required to perform these examinations is significantly less expensive than modern color flow DUS units.
discomfort without an obvious cause, as a method of evaluating the success of an intervention, and as a method of follow-up. The test is inexpensive, painless, reproducible, and relatively easy to perform. The equipment required to perform these examinations is significantly less expensive than modern color flow DUS units.
Patients with classic symptoms of PAD may have a normal physical examination and ABI. In this setting, the astute clinician must pursue an exercise physiology study performed in a vascular diagnostic laboratory. Resting pressures are measured, and the patient is then placed on a treadmill at a constant speed and constant grade of incline (commonly 2.0 miles per hour at an incline of 12.0%). The patient is asked to report initial symptoms of limb discomfort, and then the exercise is terminated when the discomfort is limiting. Following exercise, ankle and arm pressures are again measured. A significant decrease in postexercise pressure confirms the diagnosis of PAD and also characterizes the functional limitation of the symptoms (9). When treadmill testing is unavailable, or if the patient is unable to perform treadmill walking, active pedal plantarflexion is a reliable surrogate exercise test (10).
Provocative Testing in Upper Extremity Arterial Disease
Atherosclerosis is uncommon in the upper extremity arteries other than at the origin and proximal aspect of the subclavian artery. Thoracic outlet syndrome is a common nonatherosclerotic upper extremity vascular disorder. Although there are several pathophysiologic types, the most common syndrome occurs when a cervical rib or ligament compresses the sheath encompassing the subclavian artery, vein, and brachial plexus. Although most patients present with vague neurologic symptoms, approximately 10% of patients may present with upper extremity venous thrombosis, and 2% present with arterial ischemia, either from direct compression or as a result of emboli from a proximal aneurysm (11).
The physical diagnostic test known as the Adson maneuver may detect some qualitative alteration in the radial artery pulse when compared with the neutral position. However, using digital photoplethysmography in the vascular diagnostic laboratory, arterial waveforms can be assessed in the neutral and provocative positions to demonstrate an objective reduction in arterial circulation during the Adson maneuver.
Arterial Duplex Ultrasonography
Ultrasound is the use of sound waves at frequencies higher than those that can be heard by humans (typically >20,000 cycles per second [Hz]). Commercially available ultrasound units generate frequencies of 2 to 10 million cycles per second (MHz). When an electronic voltage is transmitted to an oscillator, a crystal vibrates and emits an ultrasound beam with a defined frequency in the range of 2 to 10 MHz. The ultrasound beam hits various targets in its path (i.e., soft tissue, bone, and flowing blood) and is reflected back to the crystal (12).
Ultrasound units available today use B-mode (“brightness”) technology to provide a real-time, gray-scale image. High-frequency probes (i.e., 10 MHz) provide excellent image resolution; however, the beam attenuates rapidly and cannot penetrate depths. Low-frequency probes (i.e., 2 MHz) penetrate to visualize deeper structures, while sacrificing image resolution. DUS refers to B-mode real-time imaging and focused analysis of the velocity of flowing blood in arteries and veins.
Christian Doppler described the physics of ultrasonography by identifying the Doppler shift (13). Using variables of velocity of flowing blood, velocity of sound in tissue, the difference between the frequency of transmitted and reflected sound, and the cosine of the angle of the ultrasound beam to the direction of flowing blood, the velocity of blood in vessels can be measured. This is the basis for all vascular ultrasonography and allows modern ultrasonographers to quantitate degrees of stenosis based on the velocity of blood in various segments of vessels.
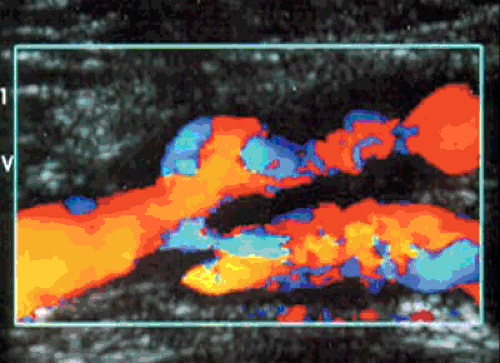
Native vessel arterial DUS is widely performed. This examination is generally accepted as a method of defining arterial stenoses or occlusions (Fig. 106.4). The sensitivity of DUS to detect occlusions and stenoses has been reported to be 95% and 92%, with specificities of 99% and 97%, respectively (14). Limitations have included tandem stenoses (15), tibial vessel imaging (16), and difficulty imaging the inflow arteries (17).
Using a 5.0- to 7.5-MHz transducer, imaging of the suprainguinal and infrainguinal arteries is performed. The vessels are studied in the sagittal plane, and Doppler velocities are obtained using a 60-degree Doppler angle. Vessels are classified into one of five categories: normal; 1% to 19% stenosis, 20% to 49% stenosis, 50% to 99% stenosis, and occlusion. The categories are determined by alterations in the Doppler waveform, as well as increasing peak systolic velocities. For a stenosis to be classified as 50% to 99%, for example, the peak systolic velocity must increase by 100% in comparison with the normal segment of artery proximal to the stenosis (18) (Table 106.2).
TABLE 106.2 Duplex Ultrasound Stenosis of Peripheral Arteries | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
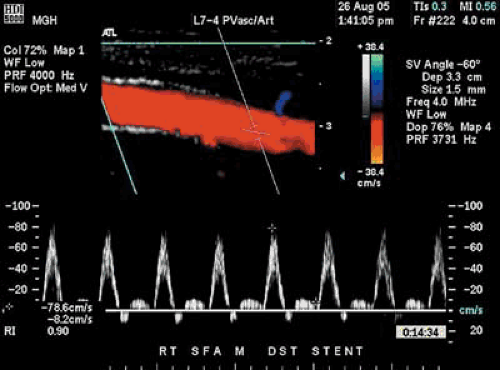
Arterial DUS has been used to guide the interventionist toward appropriate access to a lesion potentially amenable to endovascular therapy (19). This technology has also been used after endovascular therapy to determine technical success (20) and durability of the procedure (21) (Fig. 106.5). Unfortunately, it appears that DUS soon after balloon angioplasty may overestimate residual stenosis, and it may be a limitation of this technology after endovascular therapy (22).
In patients who have undergone surgical bypass graft revascularization, particularly with saphenous vein, stenoses will develop in 21% to 33% of cases. Once the graft becomes thrombosed, secondary patency rates are dismal. If the stenosis is detected and repaired before graft thrombosis, it is estimated that 80% of grafts will be salvaged (23). A well-organized graft surveillance program is crucial in preserving patency of the bypass graft (Fig. 106.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree