Nondissecting Thoracic Aneurysms
James B. Froehlich
Sanjay Rajagopalan
Gilbert R. Upchurch Jr.
David M. Williams
G. Michael Deeb
“There is no disease more conducive to clinical humility than aneurysms of the aorta.” —Sir William Osler
DEFINITION AND HISTORICAL PERSPECTIVE
The term aneurysm is derived from the Greek word “aneurysma,” which means to widen or dilate. Aneurysms may be either “fusiform,” with symmetric oblong dilatation of the aorta, or “saccular,” a circular outpouching of the aortic wall. The first descriptive account of an aneurysm was by the Roman physician-philosopher Galen around 160 A.D. Over the past three centuries, a variety of techniques such as simple ligation, wire-induced luminal thrombosis, galvanic current, and periarterial fibrosis were applied, with limited success (1,2). The modern era in the treatment of aneurysmal disease came through the work of Gross, Swan, Lam, and DeBakey, who reported successful treatment of coarctation and aneurysms of the descending thoracic aorta by using resection and replacement (3,4,5,6). The first successful aortic arch replacement under cardiopulmonary bypass was performed in 1957 by DeBakey et al. (7). Other advances such as better suture materials, prosthetic grafts, development of anticoagulants, and use of hypothermic circulatory arrest have made it possible to minimize complications on formidable operations, such as aortic arch repair.
ANATOMIC CONSIDERATIONS AND CLASSIFICATION
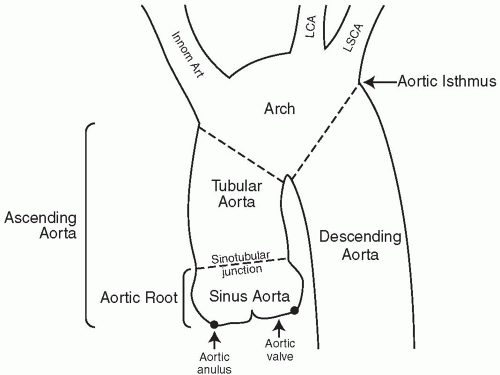
The thoracic aorta is divided into the ascending, arch, and descending portions. The ascending aorta in turn can be subdivided into the aortic root, which is the part of the aorta that extends from the level of the aortic valve to the sinotubular junction, and the tubular aorta (see Fig. 29.1). Anatomic studies of the aortic root indicate a very consistent relation among the sizes of the aortic valve leaflets, aortic sinuses, annulus, and sinotubular junction. The aorta at the level of the sinuses is the widest, measuring 3 to 3.3 cm in adults, and is usually 10% to 15% larger than the diameter of the sinotubular junction. The coronary arteries arise at this level. The aortic arch begins at the innominate artery and extends to the origin of the left subclavian artery, where it becomes the descending thoracic aorta. This transition point is the aortic isthmus. The aorta is especially vulnerable to trauma at this site because it is immobilized by the attachment to the thoracic rib cage, pleural reflections, and the left subclavian artery. The abdominal aorta continues from the thoracic aorta and bifurcates at the level of the fourth lumbar vertebra.
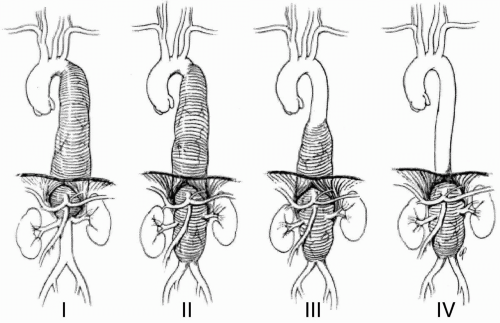
Aneurysms are classified according to their respective sites of origin as ascending, arch, or descending thoracic aneurysms. Thoracoabdominal aneurysms (TAAs), in view of their complexity, are classified
according the scheme originally devised by Crawford et al. (8) (Fig. 29.2). This classification is clinically useful in view of implications for both management and incidence of preoperative complications
according the scheme originally devised by Crawford et al. (8) (Fig. 29.2). This classification is clinically useful in view of implications for both management and incidence of preoperative complications
MECHANISMS OF ANEURYSM FORMATION
The mechanisms of aneurysm development are complex and probably involve multiple factors, such as abnormalities in matrix (genetic or acquired), excessive activity of matrix-degrading enzymes, and hemodynamic factors, which either alone or acting in concert may influence progressive dilation of the aorta.
Abnormalities in Extracellular Matrix
Alterations in matrix structure are fundamental to aneurysm development. A pathologic hallmark of aneurysms, especially those resulting from Marfan syndrome, is cystic medial necrosis (defined by elastic tissue damage, smooth muscle loss, and the accumulation of basophilic substance in the media). This change occurs to a mild degree as part of the normal aging process and may explain the association between age and the propensity for aneurysms. Marfan syndrome is an autosomal dominant disorder characterized by mutations in the fibrillin-1 molecule, the main protein component of the microfibril. Microfibrils are extracellular matrix structures displaying a diameter of less than 20 nm and lacking the characteristic 67-nm banding periodicity of interstitial collagen fibers. They form a meshwork in tissues into which elastin is embedded. In the wall of the proximal aorta, the presence of the elastinassociated microfibrillar network gives the aorta added elasticity and compliance. More
than 100 mutations have been identified in individuals affected with Marfan syndrome and other Marfan syndrome-related “fibrillinopathies,” such as MASS (mitral valve prolapse, aortic dilatation, and skin and skeletal manifestations), isolated ectopia lentis, annuloaortic ectasia, and neonatal Marfan syndrome (9,10). It has been demonstrated that FBN1 mutations can occur in individuals with thoracic aneurysms who otherwise do not meet any criteria for Marfan syndrome (11). This missense mutation, although rare, is associated with reduced synthesis of fibrillin-1 in dermal fibroblasts.
than 100 mutations have been identified in individuals affected with Marfan syndrome and other Marfan syndrome-related “fibrillinopathies,” such as MASS (mitral valve prolapse, aortic dilatation, and skin and skeletal manifestations), isolated ectopia lentis, annuloaortic ectasia, and neonatal Marfan syndrome (9,10). It has been demonstrated that FBN1 mutations can occur in individuals with thoracic aneurysms who otherwise do not meet any criteria for Marfan syndrome (11). This missense mutation, although rare, is associated with reduced synthesis of fibrillin-1 in dermal fibroblasts.
Recent advances have demonstrated that the most likely mechanism of vascular damage in Marfan syndrome is mediated by markedly elevated levels of transforming growth factor β (TGF-β), due to impaired TGF-β binding by the mutant fibrillin, an important function of normal fibrillin (12). Treatment of Marfan mice with a drug known to decrease TGF-β levels (losartan) normalizes the arterial wall of these animals (12). Beyond a potentially dramatic treatment for Marfan syndrome patients, this new insight has the potential to abrogate the effect of TGF-β in all aortic aneurysmal disease. The magnitude of the role of TGF-β on aneurysmal disease not due to Marfan has yet to be clarified.
Another disorder, Ehlers-Danlos syndrome type IV, the vascular type, results from mutations in the gene for type III procollagen (COL3A1). Affected patients are at risk for arterial, bowel, and uterine rupture. In this disorder, dissections of the aorta are common, but aneurysmal dilation of the aorta is relatively uncommon (13).
Excessive Activity of Matrix-degrading Enzymes
A substantial body of evidence implicates the increased expression and activation of a family of degrading enzymes called matrix metalloproteinases (MMPs) in the pathogenesis of abdominal and thoracoabdominal aneurysms. MMP-2, MMP-9, and membrane-bound MMP (MT-MMP) have so far been implicated (14,15,16,17,18). The evidence for a causative role for these enzymes comes from studies in which interruption in the
activity of these key enzymes by drugs (19,20,21) or targeted gene-disruption approaches (22) reduces aneurysm development. Correlative human studies have noted an increase in progression of aneurysm size and risk for rupture with an increase in the levels of these enzymes within the aortic wall (23,24). These findings have justified a large multicenter trial for the treatment of small aneurysms with tetracyclines, which are MMP inhibitors (21).
activity of these key enzymes by drugs (19,20,21) or targeted gene-disruption approaches (22) reduces aneurysm development. Correlative human studies have noted an increase in progression of aneurysm size and risk for rupture with an increase in the levels of these enzymes within the aortic wall (23,24). These findings have justified a large multicenter trial for the treatment of small aneurysms with tetracyclines, which are MMP inhibitors (21).
Hemodynamic Factors
With progressive dilation of the aorta, circumferential wall-shear stress, as defined by the Laplace law, increases (wall tension and diameter). The pulsatile load (dP/dT) tends to be greatest in the dilated portions (25). Thus modalities such as β-blockers that reduce dP/dT would be expected to reduce aneurysmal wall stress and the propensity for rupture (26).
ETIOLOGY
Table 29.1 summarizes the causes of thoracic aneurysms. Some of the causes listed differentially afflict selected portions of the aorta. For example, degenerative aneurysms most commonly involve the descending thoracic aorta and the adjacent abdominal aorta, whereas Marfan syndrome tends to involve the aortic root and tubular ascending aorta. In a small percentage of patients, aneurysms develop that are (approximately 1% each type) related to infection (mycotic aneurysm) or previous inflammatory aortitis. Infective aneurysms may involve any location and may arise through the hematogenous seeding of atherosclerotic plaque, with development of focal aortitis and formation of a false aneurysm. Aneurysms associated with blunt trauma most frequently involve the proximal descending aorta in the region of the isthmus. Dilation of the aorta distal to a congenital bicuspid aortic valve or a segment of coarctation may result from hemodynamic flow alterations distal to the stenosis.
TABLE 29.1. Causes of nondissecting thoracic aneurysm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
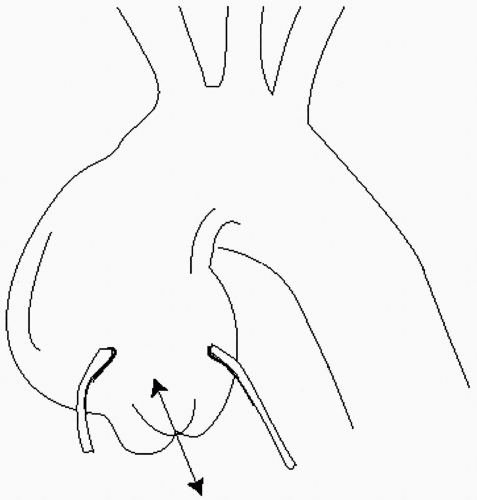
Annuloaortic ectasia is a subset of ascending aortic aneurysms that is characterized by progressive dilation of the aortic root, stretching of the leaflets, and functional aortic regurgitation (Fig. 29.3). The valves themselves are structurally normal. In due course, the aortic walls become thin and are prone to dissection. Cystic medial degeneration is the pathologic hallmark of this disorder, which is believed to represent a “fibrillinopathy,” akin to Marfan syndrome. Annuloaortic ectasia is more common in men than in women, typically occurring between the fourth and sixth decades.
INCIDENCE AND CONCOMITANT CONDITIONS
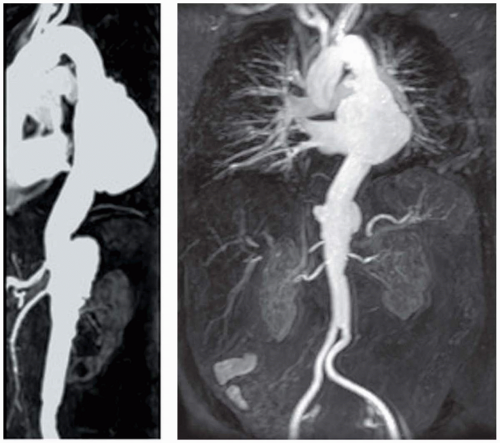
The incidence of thoracic aneurysms in the community (Rochester, Minnesota) is 5 to 10 per 100,000 patient years (27,28). In contrast to abdominal aortic aneurysms (AAAs), which demonstrate a striking predisposition for men (5:1 male-to-female ratio), thoracic aneurysms afflict men and women equally (28). The presence of a thoracic aneurysm is predictive of a higher likelihood of aneurysms in other locations. Of such affected patients, 25% harbor multiple aneurysms, in contrast to subjects with AAA, of whom only 2% harbor concomitant thoracic aneurysms (29). Figure 29.4 illustrates a large descending thoracic aneurysm in a patient who also has involvement of the suprarenal abdominal aorta. In many
instances, the appearance of a thoracic aortic aneurysm postdates that of an AAA, and, indeed, a significant proportion of patients undergoing surgery for thoracic aneurysm have undergone a prior AAA repair. In some patients this represents the de novo development of a new aneurysm; in many other instances, however, this represents the progression of previous diffuse aneurysmal disease (30). Individuals with TAA are often significantly older than patients with ascending aortic aneurysms (especially those associated with Marfan syndrome) and have concomitant coronary artery disease.
instances, the appearance of a thoracic aortic aneurysm postdates that of an AAA, and, indeed, a significant proportion of patients undergoing surgery for thoracic aneurysm have undergone a prior AAA repair. In some patients this represents the de novo development of a new aneurysm; in many other instances, however, this represents the progression of previous diffuse aneurysmal disease (30). Individuals with TAA are often significantly older than patients with ascending aortic aneurysms (especially those associated with Marfan syndrome) and have concomitant coronary artery disease.
CLINICAL PRESENTATION
The initial manifestation of a thoracic aneurysm is commonly a rupture, in which case it is uniformly fatal (31). Of patients with such aneurysms, 25% are treated on an emergency basis; approximately half are treated for frank rupture (32,33). Aneurysm progression in most patients is clinically silent. However, a larger percentage of patients with thoracic aneurysms (approximately 40%) experience symptoms, in comparison with patients with AAAs (33,34). This may in part reflect the reluctance to operate on patients in whom the aneurysm is diffuse to begin with, grows even larger, and is therefore much more likely to be symptomatic. The development of abrupt severe chest or abdominal pain may signify aneurysm expansion, rupture, or acute dissection. Thoracic aneurysms may produce localized retrosternal chest pain or back pain related to erosion into the chest wall or spine. The resultant pain may be prominent and may precede the discovery of the aneurysm by several months. Unusual symptoms related to compression of contiguous structures include (a) a new onset of hoarseness, secondary to recurrent laryngeal nerve involvement; (b) cough, secondary to
erosion or compression of the tracheobronchial tree; and (c) dysphagia lusoria.
erosion or compression of the tracheobronchial tree; and (c) dysphagia lusoria.
DIAGNOSTIC STUDIES
Chest Radiography
Widening of the mediastinum and unfurling of the ascending aorta on the chest radiograph may be the first clue to the presence of an ascending aortic or aortic arch aneurysm. The chest radiograph may, however, appear completely normal in many cases of ascending aortic aneurysms and in almost all cases of descending thoracic aneurysms and TAAs. Conversely, the presence of mediastinal widening secondary to aneurysmal enlargement cannot always be differentiated from that of tumors or other enlargements of the mediastinum.
Transesopheageal Echocardiography
Transesophageal echocardiography (TEE) with color-flow Doppler imaging is emerging as an invaluable initial study in the diagnosis and follow-up care of patients with ascending aortic and aortic arch aneurysmal disease, because it simultaneously provides important information on the status of the aortic valve, the aortic arch, and left ventricular function. For suspected dissection of the proximal aorta, a TEE is often the initial diagnostic modality of choice in many institutions.
Intraoperative monitoring with TEE during ascending aortic repair is helpful in the detection of early prosthetic complications and the adequacy of reparative procedures on the valve. In the follow-up of patients with composite graft replacement of the ascending aorta, TEE is excellent for monitoring the size of the graft and the anastomotic junction, as well as in the simultaneous evaluation of the prosthetic valve. Because patients with an ascending aortic or aortic arch aneurysm are at risk for recurrence of aneurysmal disease in the distal thoracic or abdominal aorta, three-dimensional contrast-enhanced magnetic resonance angiography (MRA) or computed tomographic (CT) scanning should be considered as a periodic surveillance adjunct in addition to TEE (35). For patients with descending thoracic aneurysms, TEE cannot provide complete information, because very often the aneurysm extends beyond the field of view. TEE is therefore not useful in the initial diagnosis or follow-up of patients with descending thoracic and TAA. However, in combination with CT scanning and angiography, it may be of use in monitoring placement of endovascular grafts and in the evaluation of early endoleaks (36).
Intraoperative monitoring with TEE during ascending aortic repair is helpful in the detection of early prosthetic complications and the adequacy of reparative procedures on the valve. In the follow-up of patients with composite graft replacement of the ascending aorta, TEE is excellent for monitoring the size of the graft and the anastomotic junction, as well as in the simultaneous evaluation of the prosthetic valve. Because patients with an ascending aortic or aortic arch aneurysm are at risk for recurrence of aneurysmal disease in the distal thoracic or abdominal aorta, three-dimensional contrast-enhanced magnetic resonance angiography (MRA) or computed tomographic (CT) scanning should be considered as a periodic surveillance adjunct in addition to TEE (35). For patients with descending thoracic aneurysms, TEE cannot provide complete information, because very often the aneurysm extends beyond the field of view. TEE is therefore not useful in the initial diagnosis or follow-up of patients with descending thoracic and TAA. However, in combination with CT scanning and angiography, it may be of use in monitoring placement of endovascular grafts and in the evaluation of early endoleaks (36).
Computed Tomography
CT techniques are commonly used in the diagnosis of thoracic aortic disease, owing to the widespread availability of CT scanners. They are of particular value in documenting growth rates of aneurysms and in determining timing for surgery, as well as obtaining detailed information on precise extent of the aneurysm (37). Computed tomographic angiography (CTA) combines a rapid bolus intravenous injection with a timed breathheld spiral CT acquisition during peak arterial opacification.
Three-dimensional reformatting with maximal intensity projections, curved planar reformations, and shaded surface displays afford excellent visualization of the aorta and major branch vessels. In the patient with a diagnosis of acute rupture, a non-contrast-enhanced CT scan is often the initial diagnostic test of choice, owing to its ready availability (38). Once the rupture is documented, no further studies are indicated, and prompt operative repair should be undertaken. If the rupture is not readily evident, contrast material is administered more completely to delineate the pathologic process and evaluate the possibility of other scenarios, such as dissection, dissecting intramural hematoma, or penetrating aortic ulcer. CTA is often necessary for endovascular planning and is a valuable adjunct in the follow-up of patients after surgery or endovascular graft placement. In the latter case, curved planar reformations are helpful in visualizing the internal anatomy of grafts and stents. The main disadvantage of CTA is its reliance on ionizing radiation and the use of contrast agents with its implications for renal dysfunction.
Magnetic Resonance Imaging
Magnetic resonance (MR) imaging has emerged as a powerful noninvasive tool for the assessment of the thoracic and thoracoabdominal aorta. It obviates the need for iodinated contrast agents and catheter-based arteriography, with their associated risks of nephrotoxicity and atheroembolism, respectively. MR protocols used in the systematic evaluation of the thoracic aorta include three-dimensional contrast-enhanced MRA (Fig. 29.4) and T1– weighted spin-echo techniques (black blood) in multiple planes to assess the extent of the aneurysm (Fig. 29.5) and provide information about the involvement of great vessels and their relation to the aneurysm. Three-dimensional contrast-enhanced MRA data-set acquisition is fast (less than 1 minute) and provides detailed images of the thoracic and abdominal aorta. Multiplanar reformations of the image data sets aid in the display of the often complex, tortuous anatomy of aneurysms. The inclusion of post-gadolinium T1-weighted images to the protocol may additionally provide information on inflammatory involvement of the vessel wall in cases of suspected aortitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree