Chapter 115
Nonatheromatous Popliteal Artery Disease
Thomas L. Forbes
The vast majority of patients experiencing lower extremity ischemic symptoms have atherosclerotic occlusive disease. However, in the absence of significant atherosclerotic risk factors, especially in younger and more active individuals, nonatheromatous causes must be considered. Adventitial cystic disease of the popliteal artery and popliteal artery entrapment syndrome are by far the most common of these rare pathologies. Similar to the more common atherosclerosis, the symptoms associated with nonatheromatous causes of popliteal artery occlusive disease range from claudication to critical limb ischemia (CLI). Given the rarity of these symptoms and the younger age of persons affected by them, these conditions are often not diagnosed in a timely fashion, resulting in prolonged disability or occasionally progression to CLI.
Embryology
The embryologic origins of the arterial system are more completely described in Chapter 2, but it is important to review some of the key features to understand the anatomy associated with pathologies affecting the popliteal artery (Fig. 115-1).
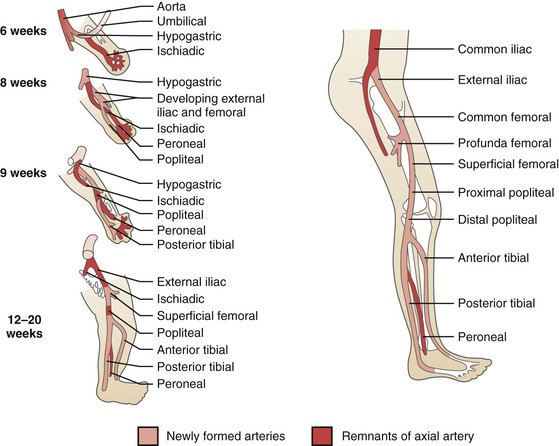
Figure 115-1 Embryologic derivation of the popliteal and other lower limb arteries. Remnants of the axial artery and arteries that develop with later differentiation are indicated. (From Levien LJ, et al: Adventitial cystic disease: a unifying hypothesis. J Vasc Surg 28:193-205, 1998.)
The lower extremity arterial system arises from two arteries—the axial and external iliac arteries—which originate from the umbilical artery. The femoral artery originates from the external iliac artery and progresses distally in the anterior compartment, whereas the axial artery elongates distally in the posterior compartment. At around 42 days of intrauterine life, the axial artery is divided into three segments, depending on its relationship to the popliteus muscle (proximal, deep, and distal); a bridging artery, the ramus communicans superius, joins the femoral artery and the proximal segment of the axial artery through the adductor hiatus. During the next week of development, the proximal component of the axial artery gives rise to a branch that runs superficial to the popliteus muscle and joins with the distal segment of the axial artery; the deep segment of the axial artery involutes. The fully developed popliteal artery results from the fusion of several arterial elements.1
Initially, both heads of the gastrocnemius muscle originate from the proximal tibia. With development, these migrate cranially along the femur to different extents. The final position of the medial head of the gastrocnemius muscle is more proximal to that of the lateral head and immediately caudal to the adductor hiatus, with the popliteal artery lying immediately lateral.1 These dynamic processes of muscle and arterial development create the potential for various anatomic variations.
Adventitial Cystic Disease
Epidemiology
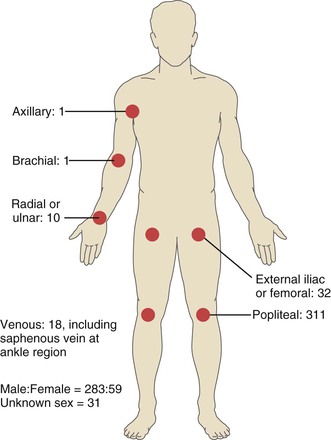
Adventitial cystic disease was first reported in 1947 with the description of a case involving the external iliac artery.2 Since then approximately 400 cases have been reported, with the popliteal artery most commonly affected (85% of cases).3 In the vast majority of cases, popliteal artery involvement is unilateral; only one report of bilateral lesions has been published.4 The next most common arteries involved are the external iliac and femoral arteries,5,6 but involvement has been noted in most of the arteries lying adjacent to joint spaces (Fig. 115-2).7 Additionally, although it is primarily and most commonly a disorder of the arterial system, isolated reports exist of adventitial cystic disease involving the iliofemoral and saphenous veins.8
Adventitial cystic disease affects males predominantly, with a male-female ratio of 5 : 1. The typical age at diagnosis is the mid-30s. Some investigators have reported a slightly older age at diagnosis in women—often in their 50s.9 It must be remembered, however, that diagnosis is often delayed, given the relatively young age of these patients and the absence of atherosclerotic risk factors. As a result, the age at which the pathologic abnormality would be apparent in an asymptomatic person is uncertain. This disease has been observed primarily in western Europe, Australia, Japan, and elsewhere in Asia. Fewer cases have been reported in North America, with the majority occurring on the East Coast.10,11 The prevalence of adventitial cystic disease has been variously reported as 1 in 1200 patients with claudication, regardless of age, and 1 in 1000 diagnostic angiograms.11 These reports include predominantly symptomatic patients, so the incidence of adventitial cystic disease in the general asymptomatic population is unknown.
Pathogenesis
Etiology
The precise cause of adventitial cystic disease remains unclear and somewhat controversial, but some clarification has occurred recently. Previously, four predominant theories of etiology and pathogenesis had been described: repetitive trauma, ganglion, systemic disorder, and developmental theories.9,11 Although convincing data to support the validity of the first three theories are scarce, they are briefly described in the following paragraphs.
Repetitive Trauma Theory.
Proponents of this theory suggest that repeated flexion and extension of the knee joint lead to chronic injury of the popliteal artery characterized by cystic degeneration.7,9,12 It is thought that this repetitive distraction movement of the popliteal artery causes intramural hemorrhage between the adventitia and media, which causes cyst formation when combined with adventitial enzymatic activity. An extension of this theory suggests that subjecting the knee joint (not the popliteal artery) to this repetitive movement and stress leads to joint degeneration and changes in the surrounding connective tissue. Consequently, these connective tissue cells secrete a substance containing hydroxyproline that results in adventitial cyst formation.12
Although the repetitive trauma theory is somewhat attractive, given its simplicity and relative intuitiveness, scientific data to support it are scarce. Repetitive trauma as a causative factor does not explain cases occurring in arteries that are not subjected to such stress or in younger patients who have not been subjected to the same duration of this stimulus as have older patients. If this theory were correct, we would expect more cases of adventitial cystic disease in more active individuals or athletes, and there would be a positive correlation between age and incidence of the disease. Neither is the case.
Ganglion Theory.
Proponents of this theory of pathogenesis have been prompted by the similar content of simple ganglions and popliteal artery cysts.7,9,13 Specifically, both types of cystic structures contain high levels of hyaluronic acid. Additionally, some support is offered by periodic case reports of synovial cystic structures and Baker’s cysts directly involving adjacent vascular structures.14 Presumably, in the case of the popliteal artery, these synovial cysts enlarge and track along arterial branches, where they implant in the adventitia of the popliteal artery itself.3 Support for this theory would include evidence of histologic similarities between the lining and chemical content of the cystic fluid in the synovium and that of popliteal artery cysts. Decades ago, several researchers failed to find any such similarities, resulting in this theory’s falling into disfavor. Specifically, fluid from these cysts has a much higher hyaluronic acid content than synovial cysts.15
Systemic Disorder Theory.
The third theory of pathogenesis for adventitial cystic disease, also lacking much supporting evidence, is that of a systemic disorder—specifically, the existence of a systemic mucinous or myxomatous degenerative condition. This theory was first postulated in 1967,16 but since then, little epidemiologic or pathologic supporting evidence has arisen, and the suspected systemic condition has never been discovered. In addition, reports of bilateral disease are exceedingly rare,4 as are cases of synchronous or metachronous cysts in different vascular locations.
Developmental Theory.
Currently, the developmental theory of pathogenesis has the greatest degree of acceptance and the most convincing body of evidence. Also known as the cellular inclusion theory, it proposes that adventitial cystic disease occurs when mesenchymal mucin-secreting cells are implanted in the adventitia of the vessel during development. Levien and Benn highlighted the embryologic development of these arteries in support of this proposal.7 They recognized that these nonaxial arteries form from vascular plexuses between 15 and 22 weeks of embryologic development adjacent to developing joints. During this time, mesenchymal cell rests forming these joints can be incorporated into closely adjacent vessels and may be responsible for subsequent cyst formation when these mesenchymal cells start to secrete mucoid material.
Connections between the knee joint capsule and an adjacent popliteal artery adventitial cyst have periodically been identified intraoperatively and by preoperative imaging.2,3,9,16–19 On the one hand, the presence of such a connection could lend support to the ganglion theory of development, with the connection representing a direct communication between the joint capsule and the arterial adventitial layer through which synovial cysts can migrate.3,17,19 Alternatively, proponents of the developmental theory claim that these communications represent a residuum of the embryologic process, when mesenchymal cells of the adjacent joint are included in the adventitia of the nearby developing artery.3,7 As mentioned previously, the biochemical analysis of these adventitial cysts does not support the ganglion theory because these cysts have a greater hyaluronic acid concentration than do synovial cysts.15
All four theories of development have been proposed as rational explanations for adventitial cystic disease at one time or another. However, the prevailing opinion and, more important, the current body of evidence largely support the developmental theory as the most rational explanation. In the absence of convincing supporting evidence, the other three theories can be viewed in the appropriate historical context.
Pathology
Popliteal artery adventitial cysts are filled with a gelatinous mucoid material. Microscopic examination reveals a simple cuboid cell lining in the adventitial layer, with a notable absence of any coexisting microscopic features of atherosclerotic disease (Fig. 115-3). Grossly, the popliteal artery may appear enlarged and sausage-like, with adhesions to adjacent structures (Fig. 115-4). The cyst is usually unilocular but can be multilocular. Cyst contents are usually clear or yellow but can be dark red following hemorrhage. At the time of operation, these cysts are apparent following incision of the adventitial layer.
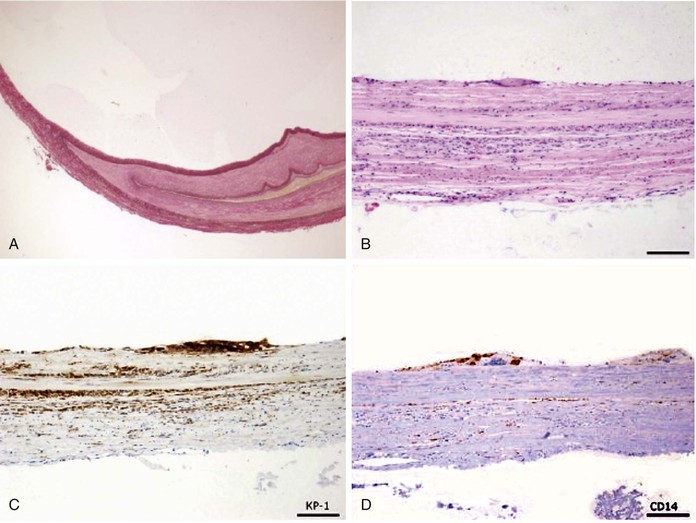
Figure 115-3 Adventitial cystic disease. A, Elastica van Gieson stain. B, Hematoxylin and eosin staining, cells lining the cysts with eosinophilic or clear cytoplasms with vacuolar or foamy structures and no basement membranes. C, CD68 stain positive cells on cyst lining. D, CD14 stain positive cells on cyst lining. (From Keiji O, et al: Rapid recurrence of cystic adventitial disease in femoral artery and an etiologic consideration for the cyst. J Vasc Surg 53:1702-1706, 2011.)
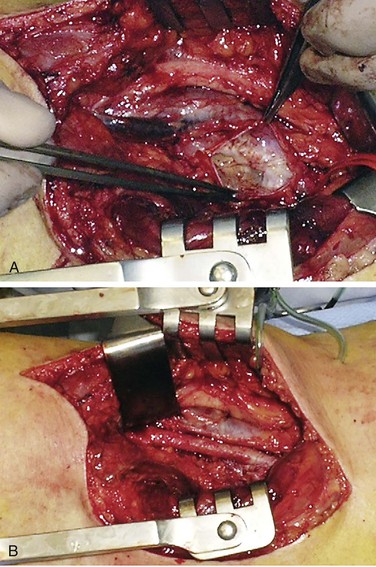
Figure 115-4 A, Adventitial cyst of popliteal artery opened and evacuated. B, Popliteal artery after cyst excision. (From van Rutte PWJ, et al: In treatment of popliteal artery cystic adventitial disease, primary bypass graft not always first choice: two case reports and a review of the literature. Eur J Vasc Endovasc Surg 42:347-454, 2011.)
Clinical Presentation
Arterial
The typical patient with adventitial cystic disease of the popliteal artery is a male in his mid-30s who complains of fairly sudden onset of short-distance calf claudication.11 The duration of symptoms is generally relatively short (weeks to a few months), and except in unusual cases, these symptoms are unilateral.4 Claudication symptoms may completely resolve for a period of time and then recur, or they may progress rapidly. Also, recovery time is often prolonged (20 minutes) compared with that of typical claudicants.20 The differential diagnosis includes popliteal artery entrapment syndrome (discussed later in this chapter), fibromuscular dysplasia, endofibrosis, and premature atherosclerosis. Given the focality of these cysts, the young age of patients, and the otherwise normal status of inflow and outflow vessels, progression to CLI is exceedingly unusual with adventitial cystic disease, although the severity of claudication can progress and become disabling. It appears that the cysts have been present and slowly enlarging for extended periods before patients enter the symptomatic phase. These enlarging cysts lead to progressive compression of the arterial lumen and can result in a “functional” occlusion of the artery without necessarily causing complete thrombosis. In fact, in cases of apparent arterial occlusion without thrombosis, evacuation of cyst contents can restore arterial patency, supporting the pathophysiology of extrinsic luminal compression with a normal intimal layer. Of course, prolonged compression of a compromised lumen can lead to popliteal artery thrombosis and a fixed occlusion. There have been anecdotal reports of spontaneous cyst resolution,21 but this appears to be extremely rare and should not be considered a feature of this disorder.
Approximately two thirds of patients present with popliteal artery stenosis rather than occlusion. On physical examination, this may be demonstrated by normal or diminished pedal pulses and by an audible bruit in the popliteal fossa. Pedal pulses that are present at rest may disappear with flexion of the knee (Ishikawa’s sign), representing a functional stenosis that proceeds to occlusion with this physical manipulation. This is in contradistinction to popliteal artery entrapment, in which case pedal pulses disappear with gastrocnemius muscle contraction caused by active plantar flexion or passive dorsiflexion of the foot.11
Venous
Cases of lower extremity venous involvement with adventitial cystic disease have been reported sporadically.8 As with arterial involvement, these venous cysts occur predominantly in males; they most commonly involve the iliofemoral venous segments, with only occasional instances of popliteal or saphenous vein involvement. The most common presentation of this rare condition is similar to that of deep venous thrombosis. Typically, a young, healthy male presents with painless swelling of the lower extremity. Venous adventitial cystic disease should be considered when there is evidence of extrinsic compression on venous duplex imaging or a filling defect on venography. The optimal method of management of venous adventitial cystic disease is not well defined, but most authors advocate operative exploration involving a venotomy with evacuation of the cyst contents, followed by cyst wall excision in the hope of minimizing the risk of recurrence.
Diagnostic Evaluation
Noninvasive studies are consistent with an isolated arterial lesion. Ankle-brachial indices are unaffected at rest and drop following exercise. This pattern should raise the suspicion of an arterial cause of the patient’s symptoms and prompt further investigation.
As with all other arterial pathologies, we have seen a steady progression of diagnostic modalities from standard angiography and Doppler ultrasound technologies to cross-sectional imaging with computed tomography (CT) and magnetic resonance imaging (MRI). Although all these methods have advantages and disadvantages with regard to adventitial cystic disease, current recommendations advocate the use of duplex scanning followed by CT or MRI as the best diagnostic approach.11
Noninvasive Testing
Duplex ultrasound should be considered the initial diagnostic tool for this disorder.22,23 The number of cysts and their dimensions can be easily evaluated; their presence, along with elevated Doppler velocities through the affected segment, can be considered diagnostic. The boundary between the cyst and the arterial lumen is depicted by a fine bright line that pulsates. Ultrasound can also differentiate these cysts from popliteal artery aneurysms by the absence of flow signals in the cysts themselves. Following intervention, duplex scanning is a useful postoperative surveillance tool to exclude cyst recurrence and residual or recurrent stenosis.
Angiography
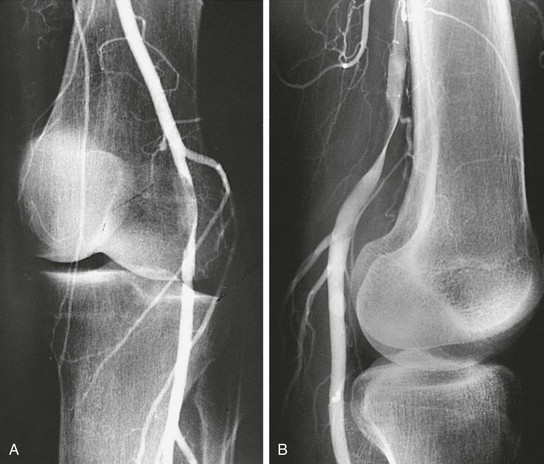
Traditionally, angiography was the diagnostic modality for adventitial cystic disease, but this has now been largely replaced by noninvasive methods. Complete popliteal artery occlusion is demonstrated with angiography in up to one third of cases, and the remaining studies demonstrate an eccentric compression of the popliteal artery lumen known as the “scimitar” sign (Figs. 115-5 and 115-6).22 Caution is warranted, however, because stenosis can be missed on anteroposterior views and may be evident only with lateral projections. In the absence of arterial thrombosis, this eccentric stenosis in the absence of poststenotic dilatation is a specific diagnostic sign of adventitial cystic disease. However, the diagnostic capability of conventional angiography is limited in the case of arterial occlusion. In this situation, angiography provides little accurate information regarding arterial wall pathology and the surrounding soft tissue.11
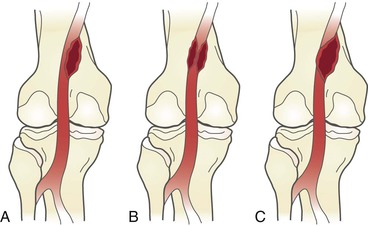
Figure 115-5 Adventitial cysts can occur in variable locations on the popliteal artery. The expanding cyst may indent the artery, resulting in the “scimitar” sign (A); encircle the artery, resulting in the “hourglass” sign (B); or completely occlude the vessel (C).
Computed Tomography and Magnetic Resonance Imaging
CT is being used more extensively in cases of popliteal artery disease (Fig. 115-7). Its main contribution to the evaluation of adventitial cystic disease is to differentiate this pathology from popliteal entrapment syndrome and aneurysmal formation, especially in cases of popliteal artery occlusion or thrombosis. CT is superior to angiography in this regard, particularly in the case of arterial occlusion; CT has the ability to demonstrate the nature of the cysts and their relationship to surrounding structures.24 It can also exclude the muscular abnormalities apparent in popliteal artery entrapment syndrome.
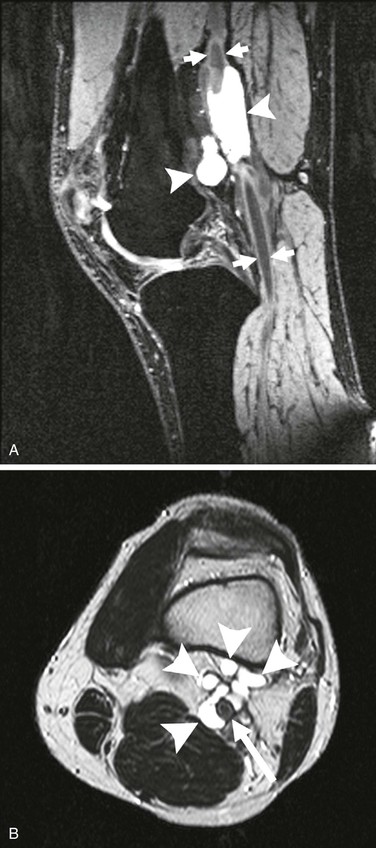
Figure 115-7 A, Cystic structures (arrowheads) in close contact with the popliteal artery (arrows). B, Cystic structures (arrowheads) in close proximity to popliteal artery (arrow). (From van Rutte PWJ, et al: In treatment of popliteal artery cystic adventitial disease, primary bypass graft not always first choice: two case reports and a review of the literature. Eur J Vasc Endovasc Surg 42:347-354, 2011.)
In the past decade, MRI has supplanted other techniques as the optimal diagnostic modality for adventitial cystic disease in the view of some investigators.25 Obvious advantages of MRI over CT include the avoidance of ionizing radiation and intravascular contrast agents. MRI clearly depicts the extent of cystic involvement, and many authors consider it essential during the planning of surgical intervention. The typical finding on MRI is an “hourglass” deformity in the popliteal artery. Technically, some authors recommend formatting MRI to include T2-weighted and gradient-echo sequences for suspected cases of adventitial cystic disease.11
Despite these strongly held beliefs, little convincing evidence supports the use of MRI over CT. However, few investigators would argue against the use of a cross-sectional imaging modality for the diagnosis of adventitial cystic disease and subsequent treatment planning. The additional information on cyst extent and anatomy provided by these techniques compared with conventional angiography can be invaluable, especially in cases of complete arterial occlusion. Duplex ultrasound remains useful as an initial diagnostic test and as an instrument of postoperative surveillance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree