Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice today. Contemporary medical treatment options include atrioventricular nodal blocking agents to control heart rates during AF, antiarrhythmic drugs aimed at maintaining normal sinus rhythm, and anticoagulation therapies to reduce stroke risk. Invasive treatment of AF has emerged because of the toxicities and lack of long-term efficacy of available antiarrhythmic medications along with the lack of improvement in symptoms for rate-controlled patients. The investigators review the evolution of the current catheter-delivered AF procedures, starting with surgical maze up to and including left atrial appendage occlusion devices. Individual catheter ablation targets, anatomic and electrophysiologic, are discussed, with a particular focus on the use of an incremental ablation target strategy dependent on the type of AF being treated. In conclusion, the history of invasive AF therapy provides a basic understanding of contemporary ablation strategies and a backdrop for the cutting-edge rhythm and stroke prevention therapies of today.
Atrial fibrillation (AF) is the most frequently encountered arrhythmia in modern medicine. Its prevalence in the adult population reaches 1.0% and increases with age, affecting an estimated 2.2 million adults in the United States, with this number projected to climb to 5.6 million by 2050. With AF carrying significant stroke and mortality risk, the increasing AF incidence has become a real health, social, and economic problem.
Contemporary medical treatment options include atrioventricular (AV) nodal blocking agents to control heart rates during AF, antiarrhythmic drugs aimed at maintaining normal sinus rhythm, and anticoagulation therapies to reduce thromboembolic risk. Invasive therapeutic options, including AF radiofrequency ablation and/or AV node ablation combined with cardiac resynchronization therapy, have found increasing use because of the toxicities and lack of long-term efficacy for maintenance of sinus rhythm of available antiarrhythmic medications. In this review, we discuss invasive, nonpharmacologic management of AF, initially taking a historic perspective, followed by a focus on contemporary therapies and cutting-edge technologies.
Surgical Therapies for Atrial Fibrillation: A Historic Focus
Surgical therapies for AF have proved successful even without a complete understanding of its underlying pathophysiology. The multiple wavelet hypothesis, proposed by Moe and Abildskov, with scientific grounding provided by the additional work of Allessie et al, formed the foundation upon which the initial surgical AF treatments were built.
In 1980, Williams et al described the first attempt at the surgical treatment of AF, termed left atrial (LA) isolation, initially performed for recalcitrant atrial tachycardias and subsequently adapted for the treatment of AF. The procedure essentially jailed the atrial fibrillatory waves in the left atrium, thus allowing sinus depolarization to control the heart. With the left atrium in permanent AF, anticoagulation was still required to minimize stroke risk while allowing control of AF.
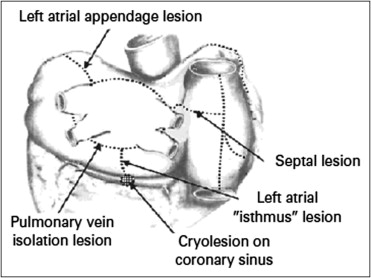
Using an understanding of the electrophysiologic mechanism of AF with multiple chaotic reentrant circuits resulting in atrial asystole and elevated thromboembolic risk, a surgical procedure was designed by Cox et al that, in a mazelike manner, aimed to interrupt all potential atrial circuits, currently known as the maze I procedure. The procedure included biatrial excisions, incisions encircling the pulmonary veins, extensive incisions starting in the right atrium and extending across the fossa ovalis, incisions from the inferior aspect of the pulmonary veins to the level of the mitral valve annulus, and finally cryoablation within the coronary sinus. Follow-up of patients after maze I revealed significant chronotropic incompetence, believed to be the result of incisions placed near the sinus node. The procedure was modified to exclude these incisions along with additional minor changes into the maze III procedure ( Figure 1 ), resulting in a dramatic improvement in the hemodynamic tolerance and short-term arrhythmic results of the procedure.
Shortly thereafter, Cox reported experience with a cohort of 308 patients who had undergone the maze III procedure, with an operative mortality of 3% and an immediate postoperative AF incidence of 37%. Subsequently, 8.5-year follow-up data on 178 patients who had undergone the maze III procedure showed that 93% remained arrhythmia free without the aid of antiarrhythmic medication. These data are limited by the method of follow-up being limited to random electrocardiographic studies or patient symptoms associated with AF, indicating that such a high success rate is not comparable to contemporary AF ablation trials that typically use more rigorous assessment of AF recurrence. Interestingly, a very low perioperative neurologic event rate (0.7%) was also described, which was thought to be due to closure of the LA appendage at the time of surgery.
Despite its published success, the maze III procedure was very complicated, involved cardiopulmonary bypass with long procedure times, and as a result did not gain widespread use. However, it did plant the seed for the development of the minimally invasive techniques that are used today. Shortly after the initial description of LA isolation, a catheter-based ablation of the His bundle using catheter fulguration was described by Scheinman et al that, with multiple evolutionary revisions, is the basis for the modern catheter-delivered minimally invasive form of the surgical maze procedure.
Contemporary Catheter Ablation
The maze procedures provided the basic framework for the contemporary invasive management of AF: surgical lines of electrical block within the atrium could return the patient to normal sinus rhythm and prevent the recurrence of AF. However, when subsequent catheter ablation approaches based on the early surgical approaches were attempted, the success rate was disappointing and accompanied by a high complication rate. What the maze procedures and their catheter-based recreations initially lacked was therapy targeting the correct underlying pathophysiology and inciting mechanism of AF.
Haïssaguerre et al in 1998 were the first to describe the “focal source hypothesis” of electrical activation responsible for the development of AF that involved atrial ectopic beats originating within the pulmonary veins. This study included 45 patients with frequent episodes of AF refractory to drug therapy and involved extensive mapping during spontaneous initiation of AF using multiple-electrode catheters. Overwhelming majorities (94%) of the inciting atrial ectopic beats were localized to the pulmonary veins with the remainder in the right and/or left atrium. Although often requiring >1 ablation procedure, ablation of the earliest ectopic foci was successful in 85%, with 62% of the entire population experiencing complete elimination of AF at a median of 7 months of follow-up.
The observation that AF could be triggered from ectopic activity originating from the pulmonary veins with ablation of the ectopic activity eliminating AF shifted the emphasis of catheter-delivered ablation away from maze-based linear lesions toward treating and isolating focal ectopic AF sources. Currently, 3 ablation strategies are used to address the focal source hypothesis of AF generation: (1) pulmonary vein isolation (PVI), (2) linear atrial lesions, and (3) complex fractionated atrial electrographic (CFAE) ablation. All of these strategies have gained widespread acceptance initially in the treatment of paroxysmal AF, followed more recently in those with chronic and heart failure–associated AF. PVI and the commonly used linear ablation lines are represented in a 3-dimensional LA map displayed in Figure 2 .
Currently, the mainstay of ablation therapy for paroxysmal AF is PVI, with electrical evidence of isolation providing maintenance of sinus rhythm in 60% to 85% of patients. Targeting CFAE ablation and or adding linear lesions can increase the rate of normal sinus rhythm maintenance to 91% at 18 months. However, long-term follow-up data after a single AF ablation have not been as promising, with arrhythmia-free survival in 29% to 53% of patients at 5 years and consequently a need for multiple repeat ablation procedures or persistent antiarrhythmic drug therapy to achieve rhythm control.
For patients with persistent or permanent AF, LA substrate modification is almost universally applied in addition to PVI, because PVI alone results in a much lower rate of freedom from AF than with the addition of substrate modification. In 1 randomized study of patients with persistent or permanent AF, the addition of LA linear lesions (LA roof line and a mitral isthmus line) improved outcomes in patients at 16-month follow-up from 20% with PVI alone to 69% with PVI and substrate modification.
All the ablative approaches within the persistent AF population use a technical end point, that is, completion of PVI or linear block, with the procedure commonly terminated by either pharmacologic or direct-current cardioversion, with consistently inducible AF in most patients at the end of the index procedure. This stands in contrast to paroxysmal AF, in which noninducibility of AF is typically the procedural end point and achieved in most patients with higher rates of success. When termination of AF by radiofrequency ablation is used as an end point in the persistent AF population, a better clinical outcome is achieved, with 1-year rates of freedom from AF considerably greater (50%) than those in whom sinus rhythm was not restored during the ablation procedure (13%). Often, a stepwise approach in which several strategies are combined (PVI, targeting of fractionated potentials, and linear lesions) is used, resulting in increased success in maintaining sinus rhythm in the medium term with recovery of atrial mechanical function in the paroxysmal as well as the persistent AF populations. Long-term data regarding the success of 1 or multiple AF ablation procedures within the persistent or permanent AF population is limited, with an ongoing need for randomized controlled trials using uniform end points accompanied by long-term follow-up.
Patients with AF and heart failure are often considered a special population. AF in heart failure is often accompanied by chronic structural atrial changes, making its medical and interventional management difficult, typically resulting in persistent or permanent forms of AF. Hsu et al assessed the utility of PVI in patients with some degree of left ventricular (LV) dysfunction (LV ejection fraction <45%) with symptoms of heart failure versus controls without LV dysfunction or heart failure who underwent PVI for drug refractory AF. After a mean of 12 months of follow-up, 78% of the patients with congestive heart failure and 84% of the controls remained in sinus rhythm (p = 0.34) (69% and 71%, respectively, were in sinus rhythm without the administration of antiarrhythmic drugs). The patients with congestive heart failure had significant improvements in LV function (LVEF increase 21%, p <0.001), LV dimensions, exercise capacity, and quality of life as well as reductions in symptoms. Ejection fractions improved significantly not only in patients without concurrent structural heart disease (24%, p <0.001) and those with inadequate rate control before ablation (23%, p <0.001) but also in those with coexisting heart disease (16%, p <0.001) and adequate rate control before ablation (17%, p <0.001). As mentioned previously, patients with AF in heart failure often have significant adverse LA remodeling that necessitates the use of multiple ablation strategies, with PVI as a base initial strategy followed by CFAE and linear ablation with the potential for repeat ablation procedures to restore sinus rhythm.
A number of AF procedural complications are commonly encountered in clinical practice. In an analysis of >1,600 patients after radiofrequency ablation of AF by Baman et al, vascular access complications were seen in 31 patients (1.9%), pericardial tamponade in 20 (1.2%), thromboembolic events (CVA) in 4 (0.2%), deep venous thrombosis in 1 (<0.01%), and pulmonary vein stenosis in 1 (<0.01%). On multivariate analysis, female gender (odds ratio 2.27, 95% confidence interval 1.31 to 2.57, p <0.01) and procedures performed in July or August (odds ratio 2.10, 95% confidence interval 1.16 to 3.80, p = 0.01) were independent predictors of any complication. Although uncommon, tamponade, CVA, and pulmonary venous stenosis are potentially morbid and are necessary considerations before procedure performance.
Contemporary Catheter Ablation
The maze procedures provided the basic framework for the contemporary invasive management of AF: surgical lines of electrical block within the atrium could return the patient to normal sinus rhythm and prevent the recurrence of AF. However, when subsequent catheter ablation approaches based on the early surgical approaches were attempted, the success rate was disappointing and accompanied by a high complication rate. What the maze procedures and their catheter-based recreations initially lacked was therapy targeting the correct underlying pathophysiology and inciting mechanism of AF.
Haïssaguerre et al in 1998 were the first to describe the “focal source hypothesis” of electrical activation responsible for the development of AF that involved atrial ectopic beats originating within the pulmonary veins. This study included 45 patients with frequent episodes of AF refractory to drug therapy and involved extensive mapping during spontaneous initiation of AF using multiple-electrode catheters. Overwhelming majorities (94%) of the inciting atrial ectopic beats were localized to the pulmonary veins with the remainder in the right and/or left atrium. Although often requiring >1 ablation procedure, ablation of the earliest ectopic foci was successful in 85%, with 62% of the entire population experiencing complete elimination of AF at a median of 7 months of follow-up.
The observation that AF could be triggered from ectopic activity originating from the pulmonary veins with ablation of the ectopic activity eliminating AF shifted the emphasis of catheter-delivered ablation away from maze-based linear lesions toward treating and isolating focal ectopic AF sources. Currently, 3 ablation strategies are used to address the focal source hypothesis of AF generation: (1) pulmonary vein isolation (PVI), (2) linear atrial lesions, and (3) complex fractionated atrial electrographic (CFAE) ablation. All of these strategies have gained widespread acceptance initially in the treatment of paroxysmal AF, followed more recently in those with chronic and heart failure–associated AF. PVI and the commonly used linear ablation lines are represented in a 3-dimensional LA map displayed in Figure 2 .
Currently, the mainstay of ablation therapy for paroxysmal AF is PVI, with electrical evidence of isolation providing maintenance of sinus rhythm in 60% to 85% of patients. Targeting CFAE ablation and or adding linear lesions can increase the rate of normal sinus rhythm maintenance to 91% at 18 months. However, long-term follow-up data after a single AF ablation have not been as promising, with arrhythmia-free survival in 29% to 53% of patients at 5 years and consequently a need for multiple repeat ablation procedures or persistent antiarrhythmic drug therapy to achieve rhythm control.
For patients with persistent or permanent AF, LA substrate modification is almost universally applied in addition to PVI, because PVI alone results in a much lower rate of freedom from AF than with the addition of substrate modification. In 1 randomized study of patients with persistent or permanent AF, the addition of LA linear lesions (LA roof line and a mitral isthmus line) improved outcomes in patients at 16-month follow-up from 20% with PVI alone to 69% with PVI and substrate modification.
All the ablative approaches within the persistent AF population use a technical end point, that is, completion of PVI or linear block, with the procedure commonly terminated by either pharmacologic or direct-current cardioversion, with consistently inducible AF in most patients at the end of the index procedure. This stands in contrast to paroxysmal AF, in which noninducibility of AF is typically the procedural end point and achieved in most patients with higher rates of success. When termination of AF by radiofrequency ablation is used as an end point in the persistent AF population, a better clinical outcome is achieved, with 1-year rates of freedom from AF considerably greater (50%) than those in whom sinus rhythm was not restored during the ablation procedure (13%). Often, a stepwise approach in which several strategies are combined (PVI, targeting of fractionated potentials, and linear lesions) is used, resulting in increased success in maintaining sinus rhythm in the medium term with recovery of atrial mechanical function in the paroxysmal as well as the persistent AF populations. Long-term data regarding the success of 1 or multiple AF ablation procedures within the persistent or permanent AF population is limited, with an ongoing need for randomized controlled trials using uniform end points accompanied by long-term follow-up.
Patients with AF and heart failure are often considered a special population. AF in heart failure is often accompanied by chronic structural atrial changes, making its medical and interventional management difficult, typically resulting in persistent or permanent forms of AF. Hsu et al assessed the utility of PVI in patients with some degree of left ventricular (LV) dysfunction (LV ejection fraction <45%) with symptoms of heart failure versus controls without LV dysfunction or heart failure who underwent PVI for drug refractory AF. After a mean of 12 months of follow-up, 78% of the patients with congestive heart failure and 84% of the controls remained in sinus rhythm (p = 0.34) (69% and 71%, respectively, were in sinus rhythm without the administration of antiarrhythmic drugs). The patients with congestive heart failure had significant improvements in LV function (LVEF increase 21%, p <0.001), LV dimensions, exercise capacity, and quality of life as well as reductions in symptoms. Ejection fractions improved significantly not only in patients without concurrent structural heart disease (24%, p <0.001) and those with inadequate rate control before ablation (23%, p <0.001) but also in those with coexisting heart disease (16%, p <0.001) and adequate rate control before ablation (17%, p <0.001). As mentioned previously, patients with AF in heart failure often have significant adverse LA remodeling that necessitates the use of multiple ablation strategies, with PVI as a base initial strategy followed by CFAE and linear ablation with the potential for repeat ablation procedures to restore sinus rhythm.
A number of AF procedural complications are commonly encountered in clinical practice. In an analysis of >1,600 patients after radiofrequency ablation of AF by Baman et al, vascular access complications were seen in 31 patients (1.9%), pericardial tamponade in 20 (1.2%), thromboembolic events (CVA) in 4 (0.2%), deep venous thrombosis in 1 (<0.01%), and pulmonary vein stenosis in 1 (<0.01%). On multivariate analysis, female gender (odds ratio 2.27, 95% confidence interval 1.31 to 2.57, p <0.01) and procedures performed in July or August (odds ratio 2.10, 95% confidence interval 1.16 to 3.80, p = 0.01) were independent predictors of any complication. Although uncommon, tamponade, CVA, and pulmonary venous stenosis are potentially morbid and are necessary considerations before procedure performance.
Ablation Targets and Techniques for Procedural Success
PVI
Using the understanding derived from Haïssaguerre et al’s landmark 1998 report, electrical isolation of pulmonary venous ostia has become the mainstay first-line ablative therapy for AF. Uniform isolation of all pulmonary veins identified is routinely performed and confirmed using a circumferential mapping catheter positioned in the pulmonary vein ostia, as displayed fluoroscopically in Figure 3 . The pulmonary veins are commonly isolated individually or as ipsilateral pairs resulting in 1 or 2 figure-of-8 lesions depending on the patient’s precise pulmonary vein anatomy and operator preference.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


