NEONATAL RHYTHM AND DYSRHYTHMIAS
Introduction
Symptomatic neonatal arrhythmias are uncommon, supraventricular tachycardia (SVT) being the commonest among them.1–3 The myocardium and conduction system in newborns have some electrophysiologic differences from the mature hearts. These differences persist from few weeks to few months after birth until more myocardial maturity evolves.1,4
In this chapter, the author discusses the developmental changes of the neonatal heart, normal neonatal rhythm, benign and nonbenign rhythm disturbances in the neonates, and neonatal bradycardia.
Conduction Tissue and Impulse Propagation in the Newborn
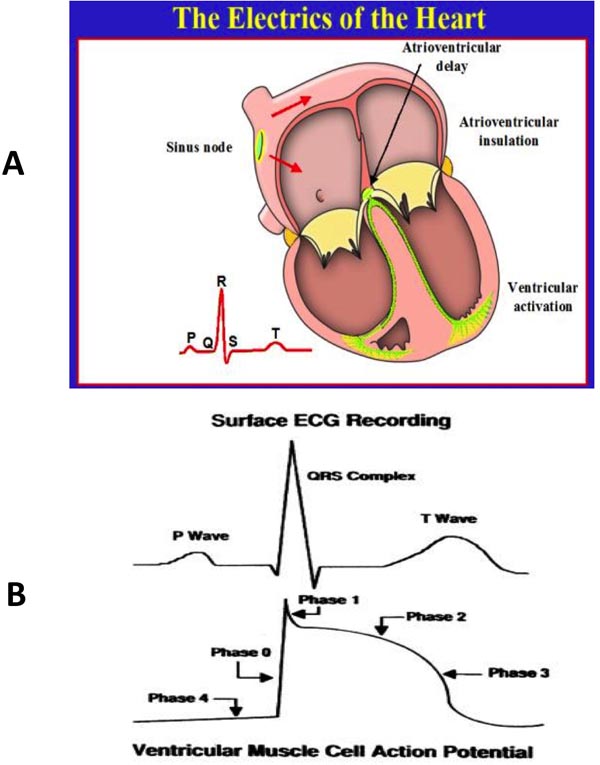
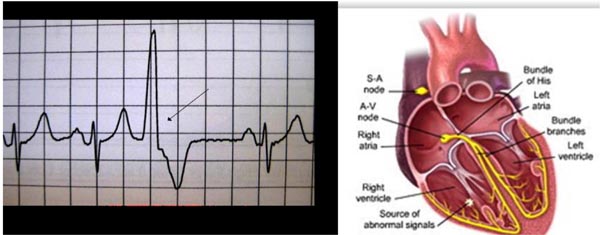
The electric propagation of the heartbeat is a fairly complex procedure that involves several ion channels and specialized tissues, which are present in the myocardial conduction tissue (sinoatrial [SA] node, atrioventricular [AV] node, His bundle, and Purkinje fibers [PF]). The action potentials of each of these specialized tissues are variable and result in different speed of conduction in these tissues (Figures 17.1 and 17.2).5–9
Figure 17.1. Panel A shows electrical activation of the heart. Panel B shows the coinciding surface QRS complex along with the action potential.
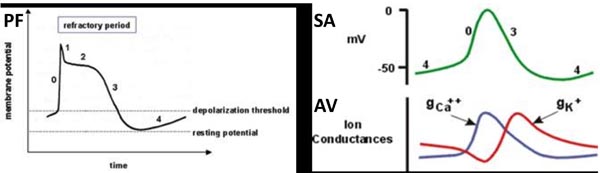
Figure 17.2. The action potential is different in PF (left panel) compared with sinoatrial (SA) and atrioventricular (AV) nodes (right panel). The myocardium in infants shows immature adrenergic and Ca2+ channel receptors altering the automaticity in their myocardium.
The AV node tissue is detected in a 35-day embryo. The formation of His bundle and PF progress with cardiac septation and mature conduction system is observed at about 8 to 9 weeks post-conception.6,7
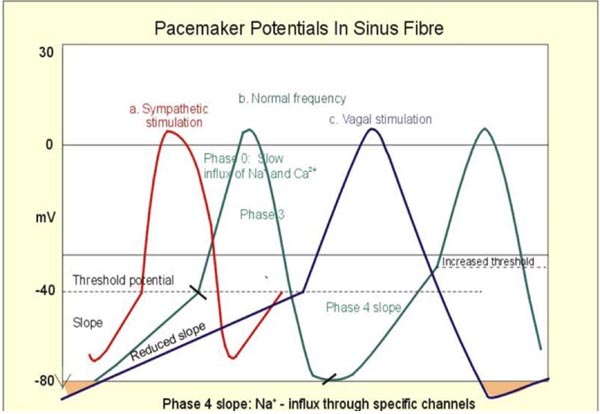
The initiation of sinus beat from the SA node is dependent on sympathetic/cholinergic input to the receptors in the SA node as well on the automaticity (phase 4 of the action potential) of these cells. Although the SA node is well developed by 37 days of embryonic heart development, full maturity of its physiology takes much longer and extend beyond the first few months of the newborn.7,8 Figure 17.3 demonstrates the effects of sympathetic and cholinergic input on phase 4 of the action potential.
Figure 17.3. Most of the benign arrhythmia occurs in newborns due to altered automaticity (phase 4 in action potential).
The neonatal myocardium is relatively deficient of the adrenergic receptors and requires higher sympathetic dose to achieve the response that is expected in older subjects. The other difference is the deficient calcium channels in the SA and AV nodal myocardium. These 2 changes will subject the neonatal myocardium to increase the effect of vagal tone.7
Arrhythmias in Newborns
Arrhythmias may be noticed incidentally in the newborn; these are bradycardia, tachycardia, or irregularity of rhythm, but the most common presentation is signs of heart failure, usually as a result of sustained tachycardia. Neonatal arrhythmia might also be an extension of fetal arrhythmias that persisted from fetal to neonatal period, which may have been noted because of the presence of heart failure in utero (hydrops fetalis).10 Use of multiple medications, high prevalence of prematurity, and increased probability of electrolyte disturbances result in a higher incidence of rhythm disturbances in the Neonatal Intensive Care Unit (NICU) setting.11
Benign Rhythm Disturbance in Newborns
Owing to the factors mentioned above, neonates are likely to have higher prevalence of spontaneous arrhythmias. Benign arrhythmias include sinus tachycardia, sinus arrhythmia, nodal or junctional rhythm, wandering atrial rhythm, premature atrial contractions (PACs), and premature ventricular contractions (PVCs).4,12,13
Sinus tachycardia Sinus tachycardia is one of the most common NICU consults to the pediatric cardiology service. Usually the patient is a premature neonate who has respiratory distress syndrome and has been placed on theophylline or caffeine for respiratory stimulation. Other medications involved are sympathomimetic (dobutamine and epinephrine) and certain antibiotics. The sinus tachycardia may reach rates of above 220/minute. Sick neonates and electrolyte disturbances can cause sinus tachycardia. The key in the diagnosis is to identify positive P waves in leads I and AVF preceding each QRS complex. Usually sinus tachycardia may slow down and warm up with vagal stimulations (passing nasogastric tube [NG]) and this will indicate the automaticity nature of this tachycardia. The treatment usually focuses upon addressing the underlying causes and weaning the medications that seem to contribute to it. Uncommonly, small doses of beta-blockers may be used in sick neonates who have low cardiac output secondary to the tachycardia, but the prime objective is to address the underlying cause. The use of beta-blockers is a last resort.
Sinus arrhythmia While sinus arrhythmias are less profound in newborns compared with older children, occasionally we see a few neonates with exaggerated sinus arrhythmia. Such episodes could be related to increased autonomic tone in some babies, particularly premature infants who are enteral fed by nasogastric or orogastric tubes. In addition, intubated babies may experience higher vagal tone and occasional sinus pauses can be observed. These pauses should be less than 2 seconds and not frequent. These sinus arrhythmias do not affect the blood pressure or tissue perfusion. Once the baby’s heart rate increases (eg, crying) these sinus arrhythmias tend to disappear. No treatment is necessary.
PACs The diagnosis of PACs relies on the following: early atrial-origin beat (P wave) compared with the preceding RR interval with change in P-wave morphology, axis, or both. Usually different PR intervals are seen (shorter if the atrial focus is near the AV node or longer if it is farther) and resets the SA node activity (post-PAC compensatory pause).
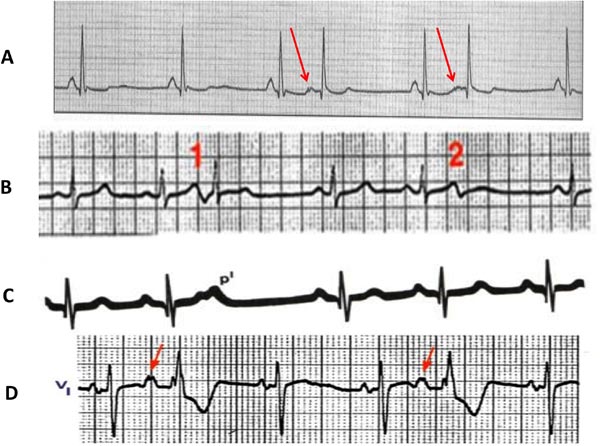
Occasionally, these PACs may be conducted to the AV node or bundle branches with aberrancy, resulting in a wider QRS that may be confused with a diagnosis of ventricular ectopy. These aberrant beats will be preceded by P waves (unlike the ventricular beats) and are usually accompanied by other PACs in the rhythm strip with narrow QRS complexes (PACs conducted without aberrancy). Other PACs may be blocked from conducting to the AV node due to its refractoriness (blocked PACs).4,11 Examples are shown in Figures 17.4 and 17.5.
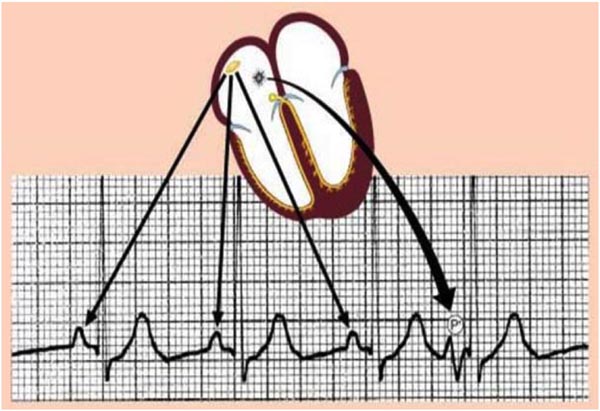
Figure 17.4. Premature atrial beat originates from the right atrium (RA): note the morphology and the axis of the PAC is different from the sinus beats.
Figure 17.5. PACs with different conductions: A: Two PACs with change in P-wave morphology (arrows) followed by compensatory pause; B: Two PACs originating from the same focus (their P morphology is the same), one of them conducted and the second got blocked from AV node conduction; C: A PAC came on top of the T wave (hiding; p1) which was blocked from conduction but reset the next sinus node beat; D: 2 PACs both conducted with aberrancy.
These PACs may be characterized as rare, occasional, and frequent during 24-hour Holter monitoring: rare if they happen less than 1% of the total beats, occasional if occur between 1% and 5%, and frequent if more than 5%. They may also manifest as atrial bigeminy pattern (alternate beats are PACs) or blocked beats from conducting to the AV node while resetting the sinus node causing bradycardia. They are the commonest arrhythmia in neonates. Kohut et al14 performed 24-hour Holter on 62 premature babies of <2500 g and found that 24 babies (~40%) had PACs of more than 5 per hour. Massin et al15 studied 264 normal subjects with 24-hour monitors and found 16% of infants in the age group 0 to 2 months to have PACs. Occasionally these PACs may result from an umbilical venous catheter placed deep into the atrium (a careful assessment by x-ray can point the cause) and slight withdrawal of this line will prevent their occurrence.
PACs in newborns have excellent prognosis with complete resolution in 90% by 3 to 6 months of age. Rarely do they need to be treated with beta-blockers if they are frequent and resulting in prolonged bradycardia secondary to atrial bigeminy and resetting the SA node.
PVCs PVCs occur significantly less frequently than PACs in the newborns. Criteria to establish the diagnosis of PVCs are as follows: they usually have wide QRS complexes, occur earlier than the next sinus beat, and are not preceded by a P wave (Figure 17.6). Usually they will not reset the sinus node (unless there is a retrograde conduction of the PVC to the atrium through the AV node).
Figure 17.6: PVC originates from the RV with wide QRS complex; note that no P wave precedes the PVC.
A careful look at the T wave of the normal sinus beat (NS) preceding the PVC is helpful to exclude a PAC that overlaps the T wave (caused the T wave to be more peaked compared with the previous one) and aberrantly conducted. This is commonly missed by physicians rendering a diagnosis of PVC.
Benign PVCs usually are monomorphic in appearance; occur after the down slope of the preceding T wave (late cycle) and non-consecutive beats. Benign PVCs tend to disappear at higher sinus rate.
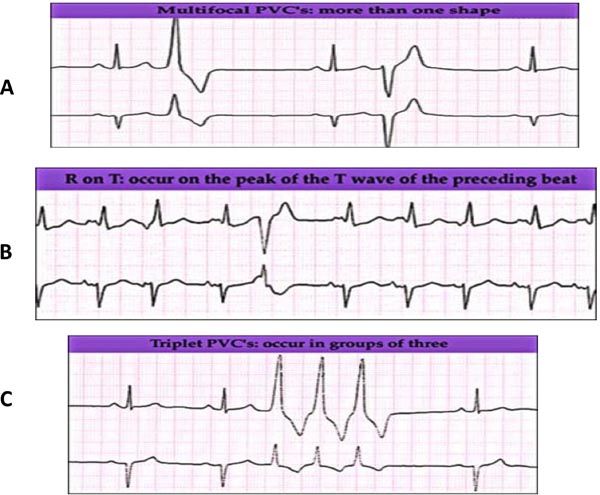
Serum electrolytes imbalance (especially calcium, potassium, and magnesium) with metabolic acidosis are common causes of PVCs in the NICU. There is a greater concern about these PVCs if they are associated with genetic channelopathy or in postoperative cardiac neonates. PVCs that are not likely to be benign in nature, such as multimorphology, early-cycle with R on T phenomena that may initiate ventricular fibrillation (VF) and consecutive beats (triplet) are shown in Figure 17.7.
Figure 17.7. Features of PVCs that are less likely to be benign in nature: A: Multi-focal morphology; B: Early-cycle with R on T phenomena that may initiate VF; and C: consecutive beats (triplet).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree