NEONATAL CARDIAC EMERGENCIES: MANAGEMENT STRATEGIES
Introduction
Life-threatening emergencies involving the cardiovascular system in the newborn are numerous and intricate. Successful treatment depends upon timely and precise diagnosis of the problem and institution of suitable therapeutic procedures and transfer, if necessary, to a specialized center equipped to care for such babies routinely. These conditions may present as severe cyanosis, heart failure, lethargy and lack of spontaneous movement, arrhythmia or hypercyanotic spells (Table 14.1). In this chapter, I wish to draw attention to these cardiac emergencies in newborn babies and to describe initial management of these conditions. Management of cardiorespiratory arrest and shock are essentially similar to that in the older infant and children and will not be dealt with in this review.
Table 14.1. Cardiac emergencies in the neonate
|
General Management
During the course of recognition and investigation, avoidance of hypothermia, preservation of neutral thermal environment, surveillance and prompt treatment of hypoglycemia and hypocalcemia, monitoring acid–base status and management of metabolic acidosis with sodium bicarbonate (NaHCO3) and addressing respiratory acidosis with suction, intubation, assisted ventilation as deemed appropriate should be meticulously carried out in all neonates. In most cyanotic heart defects, FIO2 of no more than 40% is adequate because of fixed intracardiac right-to-left shunting. In certain heart defects such as hypoplastic left heart syndrome (HLHS), 100% FIO2 may be harmful to the baby by augmenting the pulmonary flow and compromising systemic perfusion. Specific treatment measures are largely dependent on the diagnosis and will be reviewed in this chapter.
Cyanosis
Cyanosis is an important manifestation of severe congenital heart defects (CHDs) in the neonate, as has been emphasized by a number of cardiologists.1–6 Central cyanosis is evident by bluish discoloration of mucous membranes and is generally more difficult to recognize in the newborn than that in older children. The easy accessibility of pulse oxymeters facilitates authentication of cyanosis easier than obtaining blood gas analysis. Furthermore, recent studies indicate usefulness of identifying cyanotic CHD by routine pulse oximetry screening of neonates (see Chapter 9).7–10 The techniques to differentiate cardiac from noncardiac cyanosis and steps used to formulate a cardiac diagnosis are discussed in Chapter 10. As stated in that chapter, assessment of pulmonary blood flow (PBF) by chest x-ray is helpful in classification of CHD babies: decreased pulmonary vascular markings, increased pulmonary vascular markings, and pulmonary venous congestion. Babies with severe cyanosis and decreased pulmonary vascular markings on chest x-ray are expected to have severe right ventricular outflow tract (RVOT) narrowing and are likely to be dependent on ductal flow for supplying pulmonary circulation. The ductus arteriosus may be kept patent by administration of prostaglandin E1 (PGE1) intravenously. Various cardiac defects with ductal-dependent PBF in which prostaglandin (PG) is useful are listed in Table 14.2A. The current recommendation is infusion of PGE1 at a dose of 0.05 to 0.1 mcg/kg/min intravenously. The chief advantage of PG use lies in its keeping neonates in a good condition while the infant is being transferred to a tertiary care center. No more than 40% of humidified oxygen is needed in infants with cyanotic CHD because they have fixed intracardiac right-to-left shunt. Once the diagnosis is established by echo-Doppler studies, a permanent way to supply PBF, such as modified Blalock–Taussig (BT) shunt11 should be performed.
Table 14.2. Ductal-dependent cardiac defects
A. Ductal-dependent pulmonary flow
B. Ductal-dependent systemic flow
|
Neonates with severe cyanosis and increased pulmonary flow are likely to have transposition of the great arteries (TGA), while babies with mild cyanosis and increased PBF are likely have HLHS, interrupted aortic arch (IAA) or coarctation of the aorta (CoA) syndrome. In all these situations (Table 14.2B), PGE1 is helpful, this time to encourage mixing (in TGA) or to perfuse systemic circulation (in HLHS, IAA, CoA or critical aortic stenosis). In TGA, additional balloon atrial septostomy12,13 may become necessary.
Neonates with severe pulmonary venous congestion on chest x-ray are likely to have infradiaphragmatic type of total anomalous pulmonary venous connection (TAPVC) and require emergent surgical correction to include anastomosis of the common pulmonary vein with the left atrium. Stabilization with high-pressure ventilation and perhaps PGE1 infusion to decompress the pulmonary circuit are useful in the management of these babies while arranging for transport and surgery.
If the cause of cyanosis is persistent fetal circulation (no evidence for CHD and presence of markedly elevated pulmonary artery [PA] pressures), it should be treated accordingly (see Chapter 16).
Further management is largely dependent on specific diagnosis made on echocardiographic studies (see Chapter 11) and is briefly reviewed in Chapter 13.
Congestive Heart Failure (CHF)
CHF in the neonate is usually related to high PBF and is more frequent with complex CHDs such as double-inlet left ventricle (DILV) (single ventricle), double-outlet right ventricle (DORV), tricuspid atresia with a large ventricular septal defect (VSD), Swiss-cheese VSD, and others, all without accompanying pulmonary stenosis. The management of CHF includes administration of inotropic agents, diuretics, and afterload-reducing agents and is similar to that of older children14,15 and will not be reviewed here except to affirm that the neonatal myocardial development is not complete and that the myocardial response to preload and afterload manipulations and inotropic agents is less than optimal.16,17 While aggressive anticongestive treatment should be started, these are not truly emergency situations. However, ductal-dependent systemic circulation such as HLHS, IAA, CoA or critical aortic stenosis (Table 14.2B) becomes a true emergency; immediate administration of intravenous PGE1, as detailed in the previous sections, is mandatory. Once the baby is stabilized with PGE1 and anticongestive measures, accurate diagnosis should be sought by careful echocardiographic studies and the treatment tailored to the specific diagnosis (Chapter 13).
Lethargy and Lack of Spontaneous Movement
Lethargy and lack of spontaneous movement in the neonate more often than not are related to sepsis. However, it may also be seen in CHD children who have marked hypoxemia seen with severe restriction of PBF (Table 14.2A), TGA with intact ventricular septum, or severe decrease in systemic perfusion (Table 14.2B). Appropriate cultures and antibiotic treatment should be rapidly started while looking into cardiac causes. If cardiac etiology is found, it may be managed as described in the 2 preceding sections.
Arrhythmia
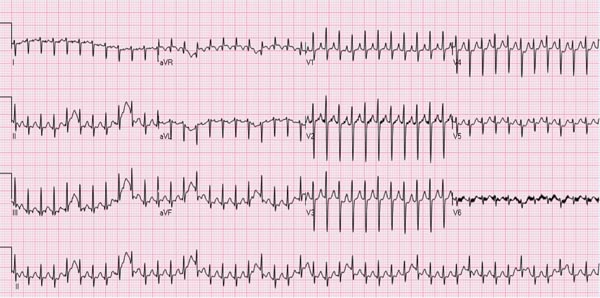
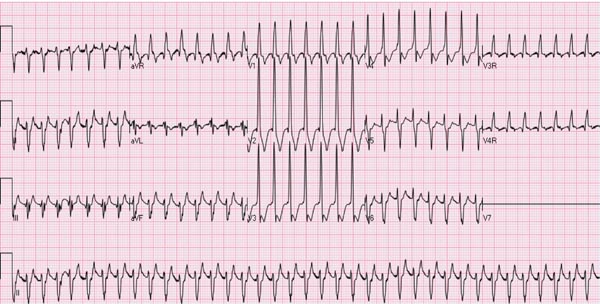
Several types of arrhythmias are seen in the neonate; most of them are benign (see Chapter 17). However, supraventricular tachycardia (SVT), the most common rhythm disorder in this age group, may cause significant hemodynamic disturbance and will be discussed in this section. SVT may also occur in fetal life and may cause hydrops fetalis (see Chapter 6). The majority of the SVT neonates do not have any other associated CHD. The heart rate in neonatal SVT varies between 220 and 280 beats per minute (bpm). The QRS complexes are usually narrow (Figure 14.1); however, wide QRS complexes may be seen, related to aberrant ventricular conduction (Figure 14.2). The most frequent (70%) mechanism underlying SVT is reentrant in type, involving an accessory pathway. The most common presentation is simple observation by the parents or other caregivers of elevated heart rate; however, these babies may also present with symptoms and signs suggestive of heart failure. Management of the episode followed by prevention of recurrence of SVT will be discussed in that order.
Figure 14.1. ECG of an infant with SVT. Note the heart rate is approximately 260 bpm; the QRS duration is very short, and no definitive P waves were seen.
Figure 14.2. ECG of an infant with SVT aberrant ventricular conduction. Note the heart rate is approximately 250 bpm, the QRS duration is prolonged, and no definitive P waves were seen.
Management of Acute Episode
Acutely ill neonates If the baby is in moderate-to-severe heart failure or has hypotension, shock, pallor, or decreased level of consciousness (neonates may have only irritability, tachypnea, and poor feeding), synchronized direct-current (DC) cardioversion with 0.5 to 1.0 W Joules/kg should be tried.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree