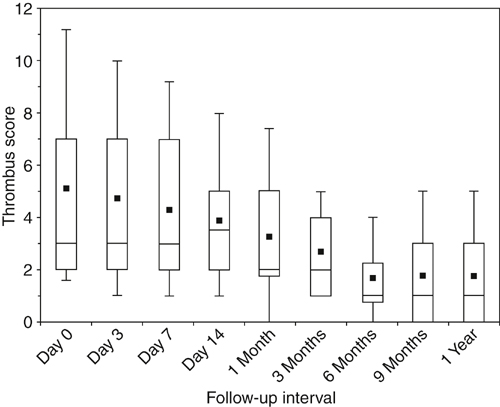
DVT is fundamentally a disease of coagulation localized to the lower extremity veins. Most of the risk factors for acute DVT are associated with some degree of hypercoagulability as a congenital, acquired, or situational risk factor (Box 1). However, most thrombi arise from multiple risk factors acting together in a synergistic fashion. Lower extremity venous thrombi originate in areas where imbalanced coagulation is localized by stasis, such as in the soleal sinuses, behind venous valve pockets, and at venous confluences. The calf veins are the most common site of origin, although 40% of proximal thrombi arise primarily in the femoral or iliac veins. Recanalization has been demonstrated clinically in several series employing serial duplex ultrasonography. Most recanalization occurs within the first 6 to 12 weeks after the acute event, and the original thrombus load is reduced by 50% to 60% within 6 to 9 months of thrombosis (Figure 1). The degree of activated coagulation and fibrinolytic inhibition are independent predictors of recanalization. Clinically, greater recanalization has been associated with thrombosis provoked by a transient risk factor, and malignancy and the presence of a permanent risk factor have been associated with less complete recanalization. In comparison to treatment with warfarin, greater recanalization has also been reported following 3 months of treatment with low-molecular-weight heparin.
Natural History of Acute Venous Thrombosis
Venous Thrombogenesis and the Early Natural History of Deep Vein Thrombosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
