Diffuse myocardial fibrosis is involved in the pathology of nonischemic cardiomyopathy (NIC). Recently, the application of native (noncontrast) myocardial T 1 measurement has been proposed as a method for characterizing diffuse interstitial fibrosis. To determine the association of native T 1 with myocardial structure and function, we prospectively studied 39 patients with NIC (defined as left ventricular ejection fraction (LVEF) ≤50% without cardiac magnetic resonance (CMR) evidence of previous infarction) and 27 subjects with normal LVEF without known overt cardiovascular disease. T 1, T 2, and extracellular volume fraction (ECV) were determined over 16 segments across the base, mid, and apical left ventricular (LV). NIC participants (57 ± 15 years) were predominantly men (74%), with a mean LVEF 34 ± 10%. Subjects with NIC had a greater native T 1 (1,131 ± 51 vs 1,069 ± 29 ms; p <0.0001), a greater ECV (0.28 ± 0.04 vs 0.25 ± 0.02, p = 0.002), and a longer myocardial T 2 (52 ± 8 vs 47 ± 5 ms; p = 0.02). After multivariate adjustment, a lower global native T 1 time in NIC was associated with a greater LVEF (β = −0.59, p = 0.0003), greater right ventricular ejection fraction (β = −0.47, p = 0.006), and smaller left atrial volume index (β = 0.51, p = 0.001). The regional distribution of native myocardial T 1 was similar in patients with and without NIC. In NIC, native myocardial T 1 is elevated in all myocardial segments, suggesting a global (not regional) abnormality of myocardial tissue composition. In conclusion, native T 1 may represent a rapid, noncontrast alternative to ECV for delineating myocardial tissue remodeling in NIC.
Diffuse myocardial fibrosis is a pathologic hallmark of the failing heart. Emerging techniques in cardiac magnetic resonance (CMR) rely on quantification of precontrast and postcontrast myocardial T 1 time to measure myocardial extracellular volume fraction (ECV), an index of diffuse interstitial fibrosis. Given that ECV is limited to patients eligible to receive gadolinium (e.g., low risk for nephrogenic systemic fibrosis) and with concomitant hematocrit, noncontrast (native) T 1 mapping has emerged as a potential biomarker of myocardial remodeling. Studies have demonstrated elevations in native T 1 in nonischemic cardiomyopathy (NIC) and its association with myocardial dysfunction. Previous work has usually sampled one area (septum) at a mid left ventricular (LV) level, assuming a diffuse disease process and lack of segmental T 1 heterogeneity observed in other myocardial disease processes. In addition, published values for native T 1 in NIC vary, possibly because of sequence-specific effects. Because histologic confirmation of native T 1 as a marker of fibrosis in NIC is limited by clinical standards against routine endomyocardial biopsy, examining native T 1 in NIC and its association with myocardial structure and function would be important to its clinical translation as a marker of cardiac remodeling. The central aims of this work were (1) to compare native T 1 in subjects with and without NIC and (2) to determine the association of native T 1 with myocardial structure and function.
Methods
We prospectively recruited 39 patients with NIC, defined as a CMR LV ejection fraction (LVEF) ≤50% without an ischemic (subendocardial) late gadolinium enhancement (LGE) pattern by CMR. Patients with known or suspected sarcoidosis, amyloidosis, anthracycline chemotherapy, or severe left-sided valvular disease were excluded. Physical examination (for weight, height, and blood pressure) was conducted at the time of CMR or at a clinic visit before CMR. Baseline demographics, medical history (including coronary artery disease risk factors), and heart failure therapies were collected by review of the medical center electronic medical record (where available) and/or discussion with study participants at the time of CMR. To provide comparison for native T 1 in a patient population without heart failure and reduced LVEF, we included data from 27 subjects as controls: (1) 12 subjects were prospectively recruited as self-reported “healthy volunteers” without any previous symptoms, the history of cardiovascular disease, or on medications for hypertension or diabetes (as determined by self-reported questionnaire) and (2) 15 subjects recruited from clinically indicated CMR with normal LVEF by CMR and absence of ischemic LGE or the history of myocardial infarction. Of note, of the total 27 subjects without NIC, the 12 subjects enrolled as “normal volunteers” did not have assessment of cardiac structure or function by CMR. Our institutional review board approved the study, and all subjects provided written informed consent.
CMR was performed on a 1.5-T magnetic resonance imaging (MRI) system (Achieva; Philips Healthcare, Best, The Netherlands). All patients were in sinus rhythm at the time of CMR. Cine images were acquired for assessment of biventricular volumes, systolic function, and mass. Vertical and horizontal long-axis and a stack of LV short-axis cine images were acquired using a breath-held steady-state free precession with retrospective electrocardiographic gating by standard society guidelines. Native T 1 mapping was performed using a free-breathing slice-interleaved T1 (STONE) sequence (repetition time/echo time= 2.8/1.4 ms, flip angle = 70°, voxel size = 2.0 to 2.1 × 2.0 to 2.1 mm 2 , slice thickness = 8 mm, slice gap = 8 mm, number of phase-encoding lines = 70, linear ordering, 10 linear ramp-up pulses, SENSE factor = 2, and bandwidth = ∼1,800 Hz/pixel). T 2 mapping was performed using a free-breathing slice-interleaved T 2 mapping sequence with the following parameters: repetition time/echo time = 2.2/1.1 ms, flip angle = 40°, voxel size = 2.5 × 2.5 mm 2 , slice thickness = 8 mm, acceleration factor = 2, acquisition window = 140 ms, multiple T 2 prep echo times = 0, 25, 35, 45, 55, 65, 75, 85, 95, and infinite. A 4-second rest period after each image was used to allow for full spin relaxation. A 2-dimensional pencil-beam navigator positioned on the right hemidiaphragm was used for prospective slice tracking (with no gating) for free-breathing native T 1 and T 2 mapping. LGE imaging was performed using a 3-dimensional imaging protocol 15 to 20 minutes after injection of 0.1 mmol/kg of gadobenate dimeglumine (MultiHance; Bracco Diagnostic Inc., Princeton, New Jersey).
Data were analyzed using a commercial workstation (Extend MR WorkSpace, version 2.3.6.3; Philips Healthcare). LV end-diastolic mass and LV and right ventricular (RV) end-diastolic and end-systolic volumes and ejection fraction were determined by manually contouring the endocardial and epicardial LV and RV borders in the short-axis orientation, respectively. Volumes and mass were then calculated using a summation of disks method. Left atrial volume and total left atrial emptying function were calculated as an index of elevated LV filling pressure in patients with NIC from 4-chamber and 2-chamber LV steady-state free precession cine imaging, as described.
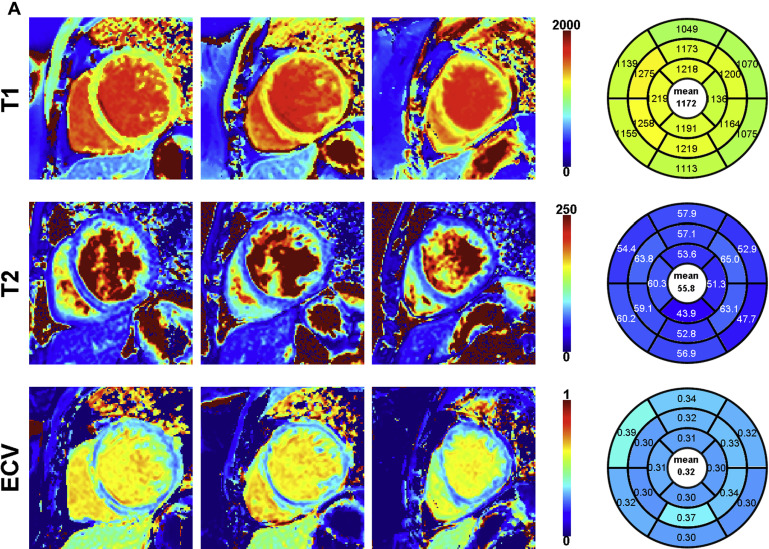
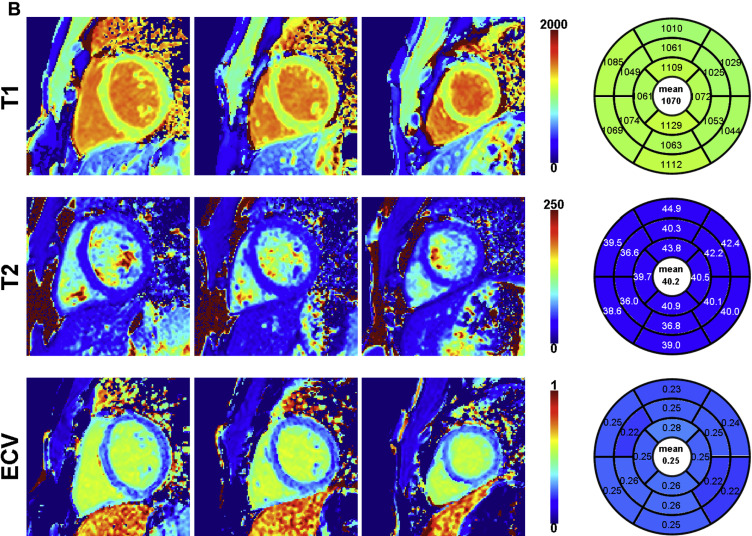
T 1 and T 2 images were exported in the Digital Imaging and Communications in Medicine (DICOM) format and were reconstructed/analyzed using an in-house platform. Residual in-plane motion and coregistration of the native T 1 scan were performed using adaptive registration of varying contrast-weighted images for improved tissue characterization technique. Voxel-wise T 1 values were finally estimated using a 2-parameter model, whereas T 2 values were estimated using a 3-parameter model. ECV maps were created using coregistered native and postcontrast T 1 maps. All parametric maps were analyzed using a 16-segment American Heart Association model. The RV insertion point and both endocardial/epicardial borders of the myocardium were manually delineated on the 3 midventricular maps, and the 16 myocardial segments were automatically generated. Two reviewers (SR and RN) reviewed all images for image integrity and exclusion of artifacts. Both were blinded to clinical and cardiac structural information.
Categorical and continuous covariates were expressed as number (percentage) or mean ± SD, as specified. We compared native T 1 , T 2 , and ECV between our non-NIC (hereafter referred to as “normal”) population and subjects with NIC using appropriate t tests. To measure association between native T 1 and important indexes of cardiac structure and function, we estimated general univariate and multivariate linear models for association between native T 1 with LV and RV ejection fraction (RVEF) and volumes and left atrial volumes and function (a surrogate for LV diastolic function and chronicity of LV end-diastolic pressure elevation). Multivariate models were adjusted for age, gender, and beta-blocker use (a surrogate for optimal heart failure therapy). Finally, we examined the segmental variation in native T 1 across all subjects, stratified by NIC status, using repeated measures analysis of variance. For linear regressions, dependent and independent variables were standardized. A 2-sided p value <0.05 was considered significant. MedCalc 12.5.0 ( http://www.medcalc.org ) and SAS 9.3 (SAS Institute, Cary, North Carolina) were used for data analysis.
Results
Demographic, clinical, and biochemical characteristics of 39 patients with NIC are summarized in Table 1 . Subjects with NIC were predominantly middle-aged men (age 57 ± 15 years; 74% men) with well-controlled blood pressure on an optimal heart failure medical regimen. Twenty-one patients with NIC (54%) had a first diagnosis of LV dysfunction noted within 1 year of CMR. Patients had well-controlled symptoms at the time of CMR (predominantly New York Heart Association functional class I to II). Overall, the population without NIC (n = 27) was younger (38 ± 15 years; 59% men). Of the 15 control participants enrolled from clinically indicated CMR scans, referral indications included evaluation of suspected cardiomyopathy (LV noncompaction, RV dysplasia, myocarditis, or hemochromatosis, n = 10); origin of ventricular arrhythmia (n = 3); and aortic valve disease (n = 2; neither had significant disease). Within our control cohort (n = 27), 15 subjects recruited from clinical CMR scans were older (47 ± 13 vs 27 ± 9 years, p = 0.0001), with a trend for greater weight (81.1 ± 19.7 vs 70.5 ± 9.1 kg, p = 0.08) and higher hypertension prevalence (27% vs 0%, p = 0.052) relative to 12 control subjects without self-reported cardiovascular disease; there was no statistical difference in gender (50% vs 67% men, in clinical vs self-reported cohort) or diabetes (0 in both).
| Characteristic | |
|---|---|
| Age (years) | 57 ± 15 |
| Male | 29 (74%) |
| Body mass index (kg/m 2 ) | 29.4 ± 6.0 |
| Systolic blood pressure (mmHg) | 119 ± 16 |
| Diastolic blood pressure (mmHg) | 71 ± 13 |
| Diabetes mellitus | 6 (15%) |
| Hyperlipidemia | 18 (46%) |
| Hypertension | 21 (54%) |
| Atrial fibrillation (history) | 7 (18%) |
| New York Heart Association functional class | 1.5 ± 0.6 |
| QRS duration (msec; N=35) | 112 ± 29 |
| Left bundle branch block (N=37) | 11 (30%) |
| Onset of cardiomyopathy within 1 year (%) | 21 (54%) |
| Medication use | |
| Beta-blockers | 32 (82%) |
| ACE inhibitor/ARB | 32 (82%) |
| Aldosterone antagonist | 11 (28%) |
| Digoxin | 4 (10%) |
Parameters of biventricular structure, function, and parametric tissue mapping are provided in Table 2 , stratified by NIC status. By definition, relative to the normal population, study participants with NIC had a decreased LVEF (p <0.0001) and a more dilated LV (LV end-diastolic volume index; p <0.0001), with greater LV mass index (p = 0.0001). Although the RVEF was slightly lower in NIC participants (p = 0.01), RV volumes were similar. Maximal left atrial volumes were similar between NIC and normal population although left atrial emptying fraction was lower in subjects with NIC (p = 0.003). Global native T 1 (an average T 1 across all available myocardial segments) was significantly higher in subjects with NIC (p <0.0001). T 2 time was slightly greater in NIC (p = 0.02) and global ECV was greater in subjects with NIC (p = 0.002).
| Cardiac structure/function ∗ | NIC | Control ∗ | P value |
|---|---|---|---|
| LV ejection fraction (%) | 34 ± 10 | 59 ± 3 | <0.0001 |
| LV end-diastolic volume index (ml/m 2 ) | 125 ± 39 | 84 ± 11 | <0.0001 |
| LV mass index (gm/m 2 ) | 68 ± 20 | 49 ± 11 | 0.0001 |
| RV ejection fraction (%) | 53 ± 12 | 59 ± 5 | 0.01 |
| RV end-diastolic volume (ml/m 2 ) | 76 ± 26 | 82 ± 12 | 0.31 |
| Maximal left atrial volume indexed (ml/m 2 ; NIC, N=38; normal, N=15) | 53 ± 23 | 44 ± 17 | 0.21 |
| Total left atrial emptying fraction (%; NIC, N=33; normal N=15) | 45 ± 14 | 55 ± 8 | 0.003 |
| Global native myocardial T 1 time (msec) | 1131 ± 51 | 1069 ± 29 | <0.0001 |
| Global myocardial T 2 time (msec; NIC, N=35; normal, N=21) | 52 ± 8 | 47 ± 5 | 0.02 |
| ECV (NIC, N=33; normal, N=20) | 0.28 ± 0.04 | 0.25 ± 0.02 | 0.002 |
| Presence of LGE | 10 (26%) | 0 (0%) | <0.0001 |
∗ Only 15 control subjects (those clinically referred for CMR) had LV, RV, and left atrial function/volumes assessed in addition to parametric tissue mapping. One patient did not receive contrast because of prohibitive glomerular filtration rate.
An example of an STONE acquisition is shown in Figure 1 . For native T 1 mapping in NIC, 56 segments (9%; of 624 segments) were excluded because of imaging artifact. For T 2 mapping acquisitions, 4 patients (10%) did not have interpretable T 2 data because of image artifacts. In the remaining 35 patients, 13 segments (2% of 560 segments) were excluded because of imaging artifact. A total of 33 patients had ECV data; 6 patients did not have ECV because of technical error (n = 1) or lack of postcontrast acquisition (n = 5). For these 33 patients, 44 segments (8% of 528 segments) were excluded because of poor image quality.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


