Myocarditis
Kathleen E. Simpson
Shafkat Anwar
Charles E. Canter
Introduction
The World Health Organization/International Society and Federation of Cardiology has defined myocarditis as an inflammatory myocardial disease diagnosed by a combination of histologic, immunologic, and immunohistochemical criteria (1). However, even as our understanding of myocarditis has evolved over the past several decades, the diagnosis of myocarditis remains challenging. This is in part due to the various observed clinical phenotypes seen in both children and adults, ranging from subclinical disease to overt cardiogenic shock. Clinicians must not only have an appropriate level of suspicion for diagnosing myocarditis, but also understand the utility of different diagnostic tools and potential therapies.
Epidemiology
The actual incidence of myocarditis is likely underestimated as some patients may have subclinical disease while others present only after sudden death. Earlier autopsy studies reported an incidence of 0.05% to 0.1% (2,3) in the general population, and up to 0.6% to 1.8% in children and young adults (3,4,5,6).
Age may also play a factor as a recent review of national data found a bimodal distribution of myocarditis, with prominent peaks in infants and mid-teenage years (7). Gender-based differences in occurrence of myocarditis have also been reported. Young adult males were found to have a higher incidence of myocarditis compared to females and older males, especially between 16 and 20 years of age (8). Younger men with acute myocarditis reportedly also have more frequent and severe evidence of fibrosis by cardiovascular magnetic resonance (CMR) compared to females and older patients (9). The exact causes for the age and gender differences are not completely understood, but may be related differences in gene expression, cellular activation, and signaling during acute and chronic disease (10).
Etiology
Causative Agents
Acute myocarditis has been associated with a wide variety of causative agents (Table 55.1). Classically, most cases of myocarditis in children and adults have been associated with viral infection secondary to commonly occurring viruses. Over the past decade, there has been a noted shift in the reported prominent causative viruses detected in myocarditis. Historically, enterovirus (particularly Coxsackieviruses A and B), and adenovirus were the predominant causative agents, but more recently, human herpes virus 6 (HHV6) and parvovirus B19 have been increasingly recognized as major causes of acute myocarditis (11,12,13). This change may be in part to advances in virus detection and increased availability of polymerase chain reaction (PCR) technology. Coxsackie B virus has been reported to be especially severe in infants with a higher rate of mortality, perhaps in part due to more diffuse expression of the coxsackievirus and adenovirus receptor (CAR) in the more immature myocardium (14). Other reported viruses include cytomegalovirus (CMV), Epstein–Barr virus (EBV), hepatitis C virus, influenza A virus, and human immunodeficiency virus (HIV) (15,16). Several cases of myocarditis were also reported associated with influenza A H1N1 strain during the 2009 outbreak (17) and the presence of associated myocarditis was found to be a significant independent predictor of mortality (18).
Myocarditis secondary to a bacterial or parasitic infection is thought to be uncommon. A wide variety of bacteria have been associated with myocarditis, including Mycoplasma pneumonia, Chlamydia pneumonia, Listeria monocytogenes, Staphylococcus, Streptococcus, Borrelia burgdorferi, Mycobacterium tuberculosis, and Corynebacterium diphtheria (19,20,21,22,23). Parasitic infections rarely lead to an eosinophilic myocarditis with noted eosinophilic predominance on histology. Chagas disease in areas with endemic Trypanosoma cruzi, such as parts of South America and Africa, is associated with not only eosinophilic myocarditis, but also an increased risk of development of apical ventricular aneurysms in affected patients (24,25). Chagas disease is increasingly seen in Latin America and cases are now appearing in the United States, especially in the Southern border states.
Eosinophilic myocarditis has also been associated with hypersensitivity reactions, autoimmune disorders, and exposure to certain toxins. Hypersensitivity myocarditis is rare in children, but may be due to exposure to vaccines or drugs, such as antibiotics and antiepileptics (26,27,28,29,30). Several autoimmune disorders are associated with significant cardiac involvement, including myocarditis, inflammatory bowel disease, hypereosinophilic syndrome (HES), sarcoidosis, systemic lupus erythmatosus (SLE), and Churg–Strauss syndrome (31,32,33). HES is characterized by endocardial fibrosis, eosinophilic inflammation of the myocardium, and mural thrombi of the right ventricle, with a poor prognosis without therapy (34). Churg–Strauss syndrome, also called eosinophilic granulomatosis with polyangiitis, involves marked inflammation of blood vessels and development of myocarditis in some patients, which is associated with relatively higher mortality (35).
Giant cell myocarditis (GCM) is a rare disease that often presents with severe, refractory heart failure. Endomyocardial biopsy (EMB) can identify the inflammatory infiltrate with multinucleated giant cells and myocyte injury (36). Death or transplant has been reported in up to 89% of patients, even with use of immunosuppressive therapy, with a median survival to death or transplant of 5.5 months after the onset of symptoms per a report of the international GCM registry (37). Treatment for GCM includes immunosuppressive therapy and transplant for disease refractory to medical therapy, although recurrence after transplant was reported in 29% of registry patients as well.
Mechanisms of Disease—A Host Response
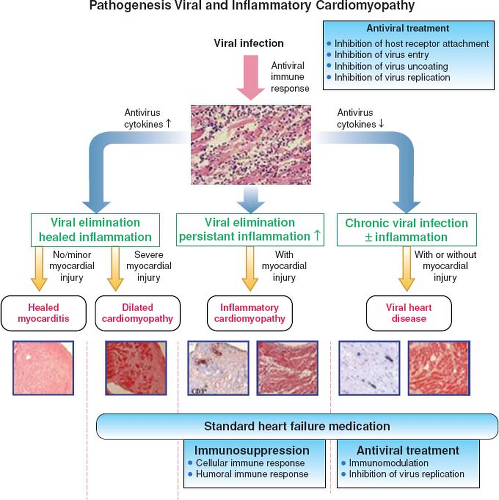
The clinical course observed in patients with myocarditis is related to balance of infectious agents and host immune interactions during
the different phases of disease (Fig. 55.1) (38,39). The initial acute phase is marked by viral infection and subsequent dissemination. The virus causes direct damage to the myocytes and also leads to host immune activation by binding to cell receptors, such as Toll-like receptors (TLRs) or CAR. These interactions trigger the release of inflammatory cytokines, including interleukin 1 and 2, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) (40). Subsequently, inflammatory cells, macrophages, and natural killer cells migrate to affected tissue in addition to further release of cytokines during the subacute phase. Other inflammatory cells, including B cells and T cells, migrate to the area of myocyte involvement and contribute to ongoing inflammation and direct tissue injury through lysis of infected myocytes. B cells are stimulated to produce virus-specific antibodies as well as autoantibodies to cardiac proteins. In the final chronic phase, there is resolution of the host immune inflammatory response with viral clearance and resolution of clinical symptoms. In some patients, there is a persistent inflammatory response with ongoing tissue damage, remodeling, and scar formation, with or without viral persistence, leading to a chronic dilated cardiomyopathy (DCM) and heart failure.
the different phases of disease (Fig. 55.1) (38,39). The initial acute phase is marked by viral infection and subsequent dissemination. The virus causes direct damage to the myocytes and also leads to host immune activation by binding to cell receptors, such as Toll-like receptors (TLRs) or CAR. These interactions trigger the release of inflammatory cytokines, including interleukin 1 and 2, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) (40). Subsequently, inflammatory cells, macrophages, and natural killer cells migrate to affected tissue in addition to further release of cytokines during the subacute phase. Other inflammatory cells, including B cells and T cells, migrate to the area of myocyte involvement and contribute to ongoing inflammation and direct tissue injury through lysis of infected myocytes. B cells are stimulated to produce virus-specific antibodies as well as autoantibodies to cardiac proteins. In the final chronic phase, there is resolution of the host immune inflammatory response with viral clearance and resolution of clinical symptoms. In some patients, there is a persistent inflammatory response with ongoing tissue damage, remodeling, and scar formation, with or without viral persistence, leading to a chronic dilated cardiomyopathy (DCM) and heart failure.
TABLE 55.1 Known Causes of Myocarditis in Children and Adults | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Autoimmunity
Autoimmune activation and production of autoantibodies against cardiac proteins have been described in patients with myocarditis and other cardiac diseases, such as Kawasaki disease (41,42,43). Cellular destruction during myocarditis leads to release of cardiac proteins, some of which have similar epitopes to viral proteins, leading to development of autoantibodies through molecular mimicry (44,45). These antibodies are thought to possibly contribute to the inflammation and cellular damage through interaction with cell receptors and release of inflammatory cytokines (45). Murine modes of myocarditis have demonstrated elevated antimyosin antibodies as well as cross-reactivity with beta-adrenergic receptors on cardiac myocytes (42,44). Beta-1 adrenergic receptor activation is known to lead increased protein-kinase A activation, which in excess has been associated with increased cellular apoptosis (46,47). Cardiac myosin has also been found to stimulate TLRs on cardiac myocytes, which have been implicated in both inhibition of viral replication but also inflammatory-mediated cell damage (48).
Other noted autoantibodies include antibodies to muscarinic-2 receptors which have been shown in rats to induce inflammation with a lymphocytic infiltrate in cardiac tissue similar to myocarditis, with subsequent development of a DCM phenotype, even in the absence of detectable antibodies to myosin or beta-adrenergic receptors (49). Anti–muscarin-2 antibodies have been shown to alter cardiac myocyte action potentials, have negative chronotropic effects and induce atrial arrhythmias, which may be a possible mechanism for conduction disease in myocarditis (49,50,51,52). In other autoimmune disorders, such as Grave disease, antibodies to beta-adrenergic receptors and muscarinic-2 receptors were found to facilitate atrial fibrillation (50). Beta-adrenergic receptor antibodies have also been associated with ventricular arrhythmias in adults with primary arrhythmias and DCM, although not specifically myocarditis (52). Antibodies to adenosine nucleotide translocator (anti-ANT), a component of heart mitochondrial membrane, have been found in patients with myocarditis and appeared to induce cardiac dysfunction in murine models (53), but levels did not correlate with extent of LV dysfunction or dilation in adults with myocarditis (54).
Clinical Features and Investigation
Clinical Phenotypes
Dilated Cardiomyopathy
The classic presentation of myocarditis includes a dilated LV with systolic dysfunction in the setting of acute-onset heart failure after a viral prodrome. Patients present with the typical
signs and symptoms of heart failure lasting several days to weeks before diagnosis. Myocarditis is recognized as a major cause of cardiomyopathy in both children and adults, accounting for 22% of new-onset LV dysfunction in a review of children with heart failure in the United Kingdom (55). Similarly, previous cardiomyopathy registry reports in the United States and Australia found that myocarditis accounted for 27% to 46% of new-onset pediatric DCM (56,57,58).
signs and symptoms of heart failure lasting several days to weeks before diagnosis. Myocarditis is recognized as a major cause of cardiomyopathy in both children and adults, accounting for 22% of new-onset LV dysfunction in a review of children with heart failure in the United Kingdom (55). Similarly, previous cardiomyopathy registry reports in the United States and Australia found that myocarditis accounted for 27% to 46% of new-onset pediatric DCM (56,57,58).
Fulminant Myocarditis
Described as a more severe form of myocarditis, fulminant myocarditis presents similarly with a history of recent viral illness followed usually within 2 to 4 weeks with sudden-onset heart failure (59,60). The magnitude of ventricular dysfunction and heart failure is more severe than typical myocarditis in most patients (61). Patients may present with cardiogenic shock, multiorgan failure, and/or life-threatening arrhythmias often requiring aggressive resuscitation, intravenous inotropes, and mechanical cardiac support (62,63). Characteristic echocardiographic findings include severe LV systolic dysfunction, increased ventricular septal thickness, and normal LV cavity dimensions (59). Despite the severity of disease during the acute illness, survival appears to be superior to classic myocarditis with reported 70% to 90% transplant-free survival at long-term follow-up (63,64).
Acute Coronary Syndrome
An increasingly recognized myocarditis phenotype mimics the presentation and findings of acute coronary syndrome (ACS), particularly in adolescent and young adult males. Patients usually present with chest pain and dyspnea of short duration with electrocardiographic (ECG) changes and elevated cardiac enzymes that are suggestive of a myocardial ischemia (65). Echocardiograms often reveal either normal function or mild decrease in ejection fraction (EF) with normal LV size (11). Vasospasm has been demonstrated in up to 70.9% of these patients and likely responsible for a substantial portion of chest pain and ECG changes seen at presentation (66). Parvovirus B19 has frequently been associated with ACS presentation (67) consistent with the apparent targeting of myocardial endothelial cells in parvovirus-mediated cardiac disease (68).
Sudden Death
Myocarditis has long been recognized as a cause of sudden death in children and adults. A pediatric autopsy study found that over half (57%) of patients with histopathology consistent with myocarditis presented with sudden death (5), while an adult autopsy study found myocarditis in 8.6% of patients who presented with nonatherosclerotic sudden death (69). Autopsy studies in athletes have reported myocarditis as a cause of approximately 3% of sudden deaths from any cause and 5% to 8% of sudden cardiac deaths (70,71,72). Myocarditis has also be recognized as a likely cause of death in infants with sudden infant death syndrome (SIDS), found in 9% of infants in one autopsy study (73). SIDS was associated with findings of positive viral PCR and/or myocardial inflammation in 43% of SIDS patients in another series (74).
Clinical Presentation and Physical Examination
In most pediatric patients, myocarditis classically presents with symptoms of acute heart failure of a relatively short duration, usually days to weeks, and a history of recent viral prodrome consisting typically of respiratory or gastrointestinal symptoms with fever (11,75,76). Respiratory symptoms are the predominant complaint in up to 80% of patients (75,76,77). Clinical examination will suggest underlying cardiac dysfunction, including tachypnea, tachycardia, hypotension, lethargy, hypoperfusion, hepatomegaly, pallor, and/or orthopnea (15,78). Children with myocarditis frequently present to the emergency department for evaluation, although misdiagnosis at initial presentation is not infrequent due to nonspecific symptoms and variable presentation (15,75).
Laboratory Evaluation
Initial evaluation typically involves a broad laboratory investigation in most cases. Patients may have elevated white blood cell counts, inflammatory markers, and liver enzymes, none of which are specific to the diagnosis of myocarditis (75). Troponin T and I levels may also be elevated as a marker of cardiac damage, but absence of increased troponin does not rule out myocarditis (79,80). Use of troponin levels have been suggested for differentiation of cardiac and noncardiac causes of pediatric chest pain, with nearly half of patients with elevated troponin levels ultimately diagnosed with cardiac disease, including 27% with myocarditis (81). However, another study found only low yield of identifying cardiac disease with use of screening during initial infectious workup in patients without cardiac symptoms (82). For pediatric patients with cardiac dysfunction of unknown etiology, a cut-off troponin T level of 0.052 to 0.088 ng/mL has been suggested to be diagnostic for acute myocarditis (80). Another study found higher troponin T and I levels in myocarditis patients compared to controls and higher levels were associated with more severe disease and mortality (79), although other studies have failed to find the same association with higher mortality (62,83). In adults, high-sensitivity troponin T was higher in myocarditis patients with positive histology and evidence of viral genome in endomyocardial tissue, but was not predictive of death or transplant (84).
B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) have been increasingly used in both adult and pediatric cardiac disease, and higher BNP levels may help differentiate between heart failure and pulmonary disease in children with respiratory distress (85). NT-proBNP may be elevated in children with myocarditis as well as those with idiopathic DCM, although levels are reportedly higher in myocarditis patients at presentation and more likely to trend down over time compared to those with idiopathic DCM (86). Elevated NT-proBNP levels have also been reported in adults with suspected myocarditis as well, with higher levels predictive of cardiac death or transplant (84). Antibodies to cardiac proteins, such as to myosin and beta-adrenergic receptors, have been demonstrated in animal models of myocarditis and antibody levels were found to be elevated in adults with myocarditis (42,43). However, the prognostic use of cardiac antibodies in children is not known and availability of antibody assays remains mainly experimental (87).
Analysis of tissues from EMB is the gold standard for investigation of possible associated infectious agent in myocarditis, with PCR replacing older techniques of cell culture or serology at many centers (88). However, not all patients with suspected myocarditis undergo EMB, and other peripheral sources have been suggested to identify causative viruses, such as serum, respiratory aspirate, urine, or stool. A small study of children with a clinical diagnosis of myocarditis or recent-onset DCM found 43% of patients had a positive blood viral PCR for a known cardiotropic virus compared to only 3% of healthy controls, although EMB PCR was not performed in a majority of the patients (89). However, another study in adults with suspected myocarditis found a lack of correlation between blood viral serology and EMB viral PCR (90). Although peripheral viral PCR is often obtained during initial evaluation, correlation with causative agents from EMB
has not been well established and evaluation is complicated by the relatively high prevalence of commonly associated viruses in the general population.
has not been well established and evaluation is complicated by the relatively high prevalence of commonly associated viruses in the general population.
Electrocardiography
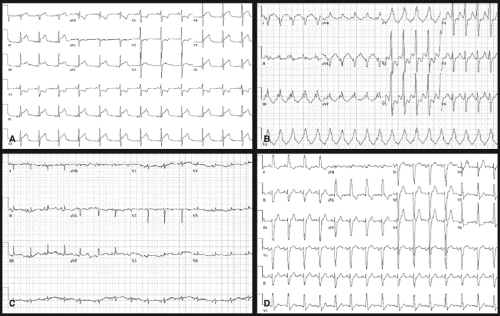
ECGs should be obtained in all patients suspected of having myocarditis and are found to be abnormal in majority of the patients (15) (Fig. 55.2). The most common abnormalities include sinus tachycardia, atrial and ventricular tachyarrhythmias, decreased QRS voltage, nonspecific ST-segment and T-wave abnormalities, and conduction delays or block (15,76,77). ECG findings may mimic those of ACS in some patients, particularly adolescents and young adults (65,75). Conduction abnormalities range from first-degree heart block to complete heart block, requiring permanent pacemaker placement in patients with refractory disease.
Radiographic Evaluation
Chest x-rays (CXR) are commonly performed due to the high frequency of respiratory symptoms at presentation. Most CXR are abnormal in patients with myocarditis (>90%), with cardiomegaly being the most common finding (15) (Fig. 55.3). Other findings include pulmonary edema, pulmonary infiltrate, or pleural effusion (75). However, cardiomegaly may not be seen in myocarditis patients who present with acute coronary-like symptoms (65).
Echocardiographic Findings
Although there are no specific echocardiographic features of myocarditis, an echocardiogram is usually obtained to assess ventricular function and left ventricular dilation, as each of these
parameters have prognostic value (91,92). An echocardiogram may also reveal an associated pericardial effusion or intracavitary thrombi, which have been noted in a number of patients with myocarditis (93).
parameters have prognostic value (91,92). An echocardiogram may also reveal an associated pericardial effusion or intracavitary thrombi, which have been noted in a number of patients with myocarditis (93).
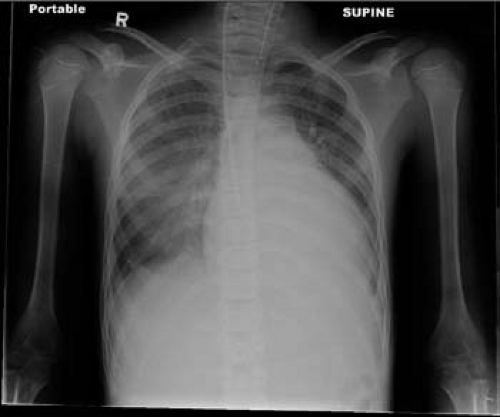 Figure 55.3 Chest x-ray from a 16-year-old male with myocarditis and heart failure. Note the cardiomegaly with pulmonary edema and pleural effusion. |
The common echocardiographic findings include ventricular dysfunction, dilation, and changes in wall thickness or wall motion abnormalities. Echocardiographic imaging can assist with distinguishing fulminant myocarditis from acute (nonfulminant) myocarditis (94). Patients with acute myocarditis usually have normal wall thickness, and may have left ventricular dilation. In contrast, those with fulminant myocarditis usually have markedly decreased systolic function, with normal chamber size and may have increased ventricular septal thickness due to myocardial edema (94). Ventricular dysfunction is not uniformly present, but may be global or regional (95). Echocardiography also helps differentiate acute and fulminant myocarditis from myocarditis with DCM, the latter presenting with markedly abnormal ventricular dilation and dysfunction (Fig. 55.4, Videos 55.1 and 55.2).
Evaluation of LV function in acute myocarditis is especially important as severity of ventricular dysfunction has been associated with increased mortality risk or requirement for heart transplantation (96). A review of children with myocarditis found that 72% had decreased EF and 64% had segmental wall abnormalities, with an initial EF<15% associated with more persistent severe cardiac failure (76). Right ventricular dysfunction has also been noted to be an independent predictor of adverse outcome in patients with biopsy-confirmed myocarditis (97). Diastolic dysfunction, including abnormal tissue velocities and strain, has been reported in children with acute myocarditis, even in the setting of normal systolic function (98). Development of diastolic dysfunction at follow-up after myocarditis has also been described in adults with heart failure symptoms, even in the setting of improved LV systolic function over the same time period (99). However, the pattern and prominence of diastolic dysfunction in children and adults over long-term follow-up has not been well described.
Acute myocarditis may resemble an ACS, usually with preserved LV function (100) and without significant coronary artery disease via angiography (67,96,101). Regional wall motion abnormalities may be noted, mainly in a noncoronary distribution, but the diagnosis of myocarditis in these patients is often confirmed with additional imaging using cardiac magnetic resonance imaging (CMR).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree