Among patients with non–ST-segment elevation acute coronary syndromes, recurrent ischemia and ventricular arrhythmias detected on continuous electrocardiographic monitoring remain common events that are associated with worse outcomes. The relative clinical significance of both events together is not well described. We determined the risk associated with ischemia (≥1 mm ST depression lasting ≥1 minutes) and ventricular tachycardia (VT) (≥4 beats) detected on 7-day continuous electrocardiographic monitoring in 6,355 patients with non–ST-segment elevation acute coronary syndromes from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non–ST-elevation Acute Coronary Syndrome–Thrombolysis In Myocardial Infarction (MERLIN-TIMI) 36 trial. The patients were categorized into 4 groups according to the presence or absence of VT and ischemia. Cardiovascular death, sudden cardiac death (SCD), myocardial infarction, and recurrent ischemia were assessed during a median follow-up of 348 days. A total of 60.0% patients had no VT or ischemia, 20.0% had VT alone, 14.7% had ischemia alone, and 5.3% had both. The patients with either VT or ischemia were at increased risk of cardiovascular outcomes. The combination of ischemia and VT identified a particularly high-risk population for cardiovascular death (10.1% vs 3.0%, p <0.001), SCD (7.8% vs 0.9%, p <0.001), and myocardial infarction (15.4% vs 6.2%, p <0.001) compared to patients with neither. The addition of arrhythmia and ischemia significantly improved the clinical model for predicting cardiovascular death or SCD (p <0.001). In patients with both ischemia and VT, 66.6% of SCD occurred within 90 days of the non–ST-segment elevation acute coronary syndromes. In conclusion, in >6,300 patients with non–ST-segment elevation acute coronary syndromes, the presence of myocardial ischemia or VT alone, and particularly in combination, was independently associated with poor cardiovascular outcomes and thus provides incremental improvement in early risk stratification.
Despite advances in the treatment of patients with acute coronary syndrome, recurrent ischemia and ventricular arrhythmias occur frequently after the initial stabilization of the acute coronary event. Continuous electrocardiographic (cECG) monitoring is a sensitive method to detect and quantify recurrent myocardial ischemia and ventricular tachycardia (VT). Several previous studies have demonstrated that the presence of either ischemia or VT detected on cECG after acute coronary syndrome is associated with poor cardiovascular outcomes. Recent analyses in >6,300 patients from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non–ST-elevation Acute Coronary Syndrome–Thrombolysis In Myocardial Infarction (MERLIN-TIMI) 36 trial demonstrated that VT or ischemia alone detected on 7-day cECG monitoring was independently associated with poor cardiovascular outcomes, including myocardial infarction, cardiovascular death (CVD), and sudden cardiac death (SCD). However, the frequency and cumulative risk of poor cardiovascular outcomes in patients with both nonsustained VT and ischemia detected on cECG remains uncertain. The objective of the present analysis was to evaluate the incremental risk associated with the presence of both nonsustained VT and ischemia after non–ST-segment elevation acute coronary syndrome and to determine whether identifying these events improves risk stratification.
Methods
In the MERLIN-TIMI 36 trial, 6,560 patients hospitalized with non–ST-segment elevation acute coronary syndrome were randomized to receive either ranolazine or placebo in addition to standard medical therapy. Eligible patients had ≥10 minutes of ischemic symptoms at rest and ≥1 of the following moderate- to high-risk features: elevated biomarkers of myocardial necrosis, ST-segment depression ≥0.1 mV, history of diabetes mellitus, or an intermediate to high TIMI risk score (≥3). The exclusion criteria relevant to the present analysis include left bundle branch block, predominant ventricular paced rhythm, significant left ventricular hypertrophy, and concurrent digoxin use. Medications that prolonged the QT interval such as class IA or III antiarrhythmic agents were prohibited. A cECG recording (Lifecard CF, Delmar Reynolds/Spacelabs, Issaqua, WA) was performed for the first 7 days after randomization in all patients to assess for ischemia and arrhythmias as a part of an efficacy and safety analysis. Analysts and cardiologists who were unaware of the treatment assignment and outcomes determined the presence of ischemia and arrhythmia in the TIMI ECG Core Laboratory.
Ischemia evaluated by cECG assessment was defined as ≥1 mm ST-segment depression lasting ≥1 minute. For the principal analyses, we defined VT as ≥4 consecutive ventricular beats at a rate >100 beats/min. In the sensitivity analyses, we also identified those patients whose longest ventricular ectopy was 3 consecutive beats, or triplets. The patients were categorized into 1 of 4 groups according to the presence or absence of VT and ischemia (VT negative/ischemia negative, VT positive/ischemia negative, VT negative/ischemia positive, and VT positive/ischemia positive).
All analyses excluded any clinical events (CVD, SCD, myocardial infarction, and recurrent ischemia) that occurred within the first 7 days during the cECG monitoring period. The mean clinical follow-up was 348 days, and all clinical end points, including CVD, SCD, myocardial infarction, and recurrent ischemia, were adjudicated by a blinded clinical events committee using the clinical narratives, discharge summaries, and autopsy reports. Recurrent ischemia was defined by electrocardiographic changes, ischemia leading to hospitalization, revascularization, and worsening angina, prompting intensification of antianginal therapy. SCD was defined according to a previously described schema as either: (1) witnessed and occurring within 60 minutes from the onset of new symptoms and in the absence of a clear cause other than cardiovascular; or (2) unwitnessed and occurring within 24 hours of being observed alive in the absence of pre-existing progressive circulatory failure or other noncardiovascular causes of death.
Brain natriuretic protein (BNP) was measured in 4,543 patients using the ADVIA Centaur (Siemens Medical Solutions, Malvern, Pennsylvania) in the TIMI Biomarker Core Laboratory (Boston, Massachusetts) using a prespecified decision limit of 80 pg/ml, based on our previous work. The TIMI risk score was calculated using the previously described method and categorized as low (0–2), moderate (3–4), or high (>4) risk. The left ventricular ejection fraction was reported by the investigators in 4,428 patients.
A total of 6,345 patients (96.7% of the entire MERLIN-TIMI 36 population) had cECG recordings that were interpretable for analysis. The hazard ratios (HRs) and 95% confidence intervals were estimated using a Cox proportional hazards regression model adjusted first for the TIMI risk score, previous myocardial infarction, previous heart failure, estimated creatinine clearance (Cockcroft-Gault), and revascularization during the index hospitalization and then stratified by the prerandomization intention to use an early invasive strategy. The model was then repeated with the inclusion of BNP and the left ventricular ejection fraction for patients in whom these variables were assessed. The event rates are presented as the Kaplan-Meier failure rates at 12 months. All analyses were performed by the TIMI study group.
Estimates of the C-statistic for the Cox regression clinical models were calculated both with and without the ECG parameters. The increased discriminative value was examined using the method described by Pencina et al and programmed in R using Harrell’s technique to determine the net reclassification improvement and the integrated discrimination improvement.
Results
Ventricular arrhythmias detected on cECG within 7 days after randomization were common with 1,603 patients (25.3%) having ≥1 episode of VT. Ischemia was detected on cECG in 1/5 of all patients (n = 1,269, 20.0%). When categorized into the 4 previously mentioned groups, 3,808 patients (60.0%) had no VT or ischemia detected on the 7-day continuous electrocardiographic monitoring, 1,268 (20.0%) had VT but no ischemia, 934 (14.7%) had ischemia but no VT, and 335 (5.3%) had both VT and ischemia detected by 7-day cECG monitoring.
The baseline characteristics of the 6,355 patients with evaluable cECG monitoring are presented in Table 1 according to their VT/ischemia group. The patients with ischemia on cECG monitoring were more likely to be elderly, women, and have a history of chronic kidney disease, heart failure, or a moderate to high TIMI risk score. Also, at presentation, they were more likely to have ST-segment depression and elevated BNP or troponin. Patients with VT were also more likely to have an elevated BNP level and troponin but had a similar TIMI risk score distribution compared to patients with neither VT nor ischemia. The use of β blockers was similar between the 4 VT/ischemia groups. Revascularization during the index procedure occurred less frequently among patients with neither VT nor ischemia.
| Characteristic | VT-Negative/Ischemia-Negative (n = 3,808) | VT-Positive/Ischemia-Negative (n = 1,268) | VT-Negative/Ischemia-Positive (n = 934) | VT-Positive/Ischemia-Positive (n = 335) | p Value |
|---|---|---|---|---|---|
| Age (years) | 62.5 ± 11.0 | 63.3 ± 11.1 | 66.4 ± 10.3 | 67.2 ± 10.5 | 0.001 |
| Women | 35.8% | 29.1% | 40.5% | 33.1% | <0.001 |
| Diabetes mellitus | 35.7% | 30.2% | 32.8% | 30.2% | 0.001 |
| Previous heart failure | 15.7% | 16.3% | 21.7% | 17.9% | <0.001 |
| Creatinine clearance <60 ml/min | 19.5% | 19.1% | 30.5% | 27.5% | <0.001 |
| Revascularization during index hospitalization | 36.8% | 44.2% | 39.8% | 49.4% | <0.001 |
| β Blocker during index hospitalization | 83.4% | 83.8% | 82.4% | 83.5% | 0.565 |
| ST-segment depression >0.1 mV on admission | 28.0% | 31.6% | 60.1% | 63.9% | <0.001 |
| Elevated troponin (>0.04 mg/L) | 60.7% | 70.2% | 69.7% | 77.3% | <0.001 |
| TIMI risk score | <0.001 | ||||
| 0–2 | 29.6% | 27.8% | 18.5% | 14.3% | |
| 3–4 | 53.1% | 53.8% | 50.9% | 47.5% | |
| 5–7 | 17.3% | 18.4% | 30.6% | 38.2% |
The presence of either VT alone or ischemia alone on 7-day cECG monitoring significantly increased the risk of CVD and SCD ( Figure 1 ) . The detection of ischemia alone was also associated with an increased risk of recurrent ischemia and myocardial infarction ( Figure 1 ). The greatest risk was observed in patients with both VT and ischemia who, compared to patients with neither ischemia nor VT, had a more than fivefold increased risk of CVD (14.8% vs 2.6%, adjusted HR 5.4, p <0.001) and a more than sixfold increased risk of SCD (7.8% vs 0.9%, adjusted HR 6.1, p <0.001).
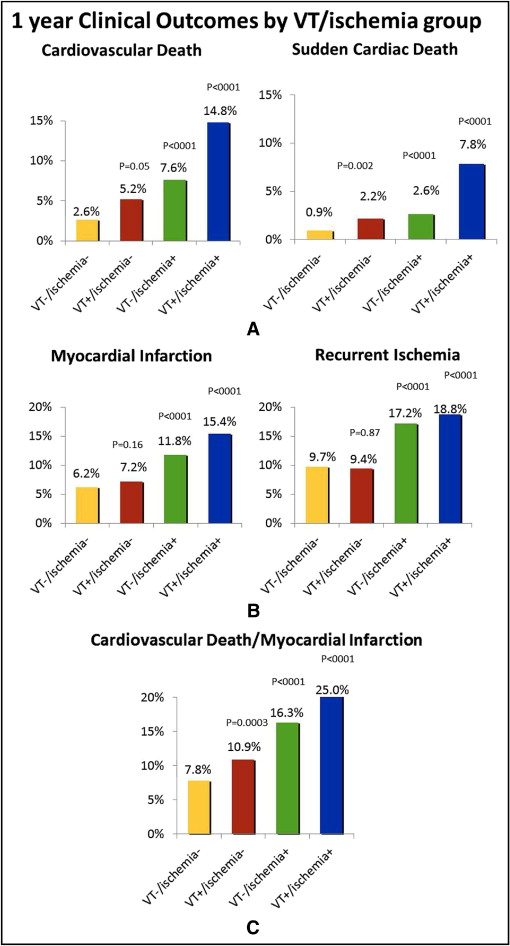
To determine the significance of shorter episodes of ventricular ectopy, we identified patients with VT lasting 3 beats only, or triplets (n = 1,978, 31.2% of all patients). In the absence of ischemia, the presence of 3 beats of VT (n = 1,590, 25%) was not associated with any increase in the risk of CVD (3.0% vs 2.3%, p = 0.206) or SCD (1.1% vs 0.8%, p = 0.349). A trend was seen toward an increased risk of CVD among patients with ischemia on cECG monitoring and triplets (n = 388, 6.2%) compared to ischemia alone (9.7% vs 6.2%, p = 0.06) but not for SCD (2.9% vs 2.3%, p = 0.206).
The cumulative incidence of CVD and SCD (a component of CVD) according to the VT/ischemia groups is presented in Figure 2 , demonstrating the stepwise gradient of risk across the groups. The risk of CVD and SCD increased within the first few weeks after the index event, with most CVDs and SCDs occurring within 90 days of the index event (73.7% of CVD and 66.6% of SCD).
In the multivariate model that included the baseline clinical characteristics, the presence of ischemia and VT detected on cECG monitoring continued to be strongly and independently associated with all cardiovascular end points, including CVD and SCD ( Table 2 ). The risk associated with both electrocardiographic parameters was on the same order of magnitude as the TIMI risk score and the other clinical variables. These relations remained robust and independent when the biomarker data and left ventricular function were added to the model. Treatment with ranolazine did not significantly modify the risk associated with ischemia or VT in relation to any of the clinical outcomes.
| Characteristic | CVD | SCD | ||
|---|---|---|---|---|
| Adjusted HR | p Value | Adjusted HR | p Value | |
| Model 1 (clinical variables) | ||||
| VT/no ischemia | 2.2 | <0.001 | 2.3 | 0.001 |
| No VT/ischemia | 2.4 | <0.001 | 2.4 | <0.001 |
| VT/ischemia | 5.4 | <0.001 | 6.5 | <0.001 |
| Moderate TIMI risk score | 1.8 | 0.01 | 1.6 | 0.14 |
| High TIMI risk score | 3.0 | <0.001 | 2.3 | 0.02 |
| No revascularization at index event | 2.0 | <0.001 | 2.0 | 0.01 |
| Creatinine clearance <60 ml/min | 2.4 | <0.001 | 1.8 | 0.003 |
| Previous myocardial infarction | 0.93 | 0.61 | 1.0 | 0.98 |
| Previous congestive heart failure | 2.1 | <0.001 | 2.3 | <0.001 |
| Ranolazine | 1.1 | 0.42 | 0.97 | 0.88 |
| Model 2 (model 1 plus left ventricular ejection fraction) | ||||
| Ejection fraction <40% | 3.2 | <0.001 | 2.8 | <0.001 |
| VT/no ischemia | 1.7 | 0.01 | 1.7 | 0.11 |
| No VT/ischemia | 2.5 | <0.001 | 2.3 | 0.01 |
| VT/ischemia | 4.4 | <0.001 | 7.3 | <0.001 |
| Model 3 (model 1 plus brain natriuretic protein) | ||||
| Brain natriuretic protein >80 pg/ml | 2.1 | <0.001 | 1.5 | 0.14 |
| VT/no ischemia | 1.6 | 0.01 | 2.3 | 0.007 |
| No VT/ischemia | 2.1 | <0.001 | 2.4 | 0.003 |
| VT/ischemia | 4.6 | <0.001 | 3.5 | <0.001 |
The incidence of ischemia and VT within multiple subgroups and the associated risk of CVD and SCD are listed in Table 3 . A steep gradient of risk was found among all subgroups, with the lowest risk among patients without VT or ischemia and the greatest for those with both. The absence of ischemia and VT in the following low-risk populations—age <65 years, no diabetes, ejection fraction ≥40%, BNP <80 pg/ml, or undetectable troponin—identified a cohort with a particularly low rate of CVD (age <65 years, 1.0%; no diabetes, 1.9%; ejection fraction ≥40, 2.0%; BNP <80 pg/ml, 0.77%; negative troponin, 0.88%) and SCD (age <65 years, 0.4; no diabetes, 0.74%; ejection fraction ≥40%, 0.85%; BNP <80 pg/ml, 0.27%; negative troponin, 0.55%). However, even among these same low-risk groups, those with VT and ischemia on cECG monitoring had particularly high risk. For example, in patients with negative troponin on admission, the incidence of CVD was 0.88% in patients with no ischemia/VT compared to 15.8% in patients with both (HR 14.1, 95% confidence interval 5.59 to 35.6, p <0.001). Among patients with an already high absolute risk of CVD, including those with a history of diabetes, a history of heart failure, a reduced left ventricular function, and elevated cardiac biomarkers, the additional risk associated with having both VT and ischemia on cECG monitoring is considerable (diabetes, 3.8% vs 18.3%; previous heart failure, 5.7% vs 32.5%; ejection fraction ≤40%, 9.9% vs 25.5%; BNP >80 pg/ml, 5.4% vs 19.7%; elevated troponin, 3.6% vs 16.7%).
| Subgroup | Patients (n) | VT/Ischemia Category | CVD | SCD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1-yr Kaplan-Meier (%) | HR adj | 95% CI | p Value | 1-yr Kaplan-Meier (%) | HR adj | 95% CI | p Value | |||
| Age (years) | ||||||||||
| <65 | 3,112 | |||||||||
| VT−/ischemia− | 1.0% | Referent | 0.4% | Referent | ||||||
| VT+/ischemia− | 3.4% | 3.6 | 2.0–6.4 | <0.001 | 1.2% | 3.5 | 1.5–8.4 | 0.004 | ||
| VT−/ischemia+ | 2.9% | 2.7 | 1.4–5.6 | 0.005 | 1.5% | 3.1 | 1.1–8.5 | 0.03 | ||
| VT+/ischemia+ | 5.6% | 6.5 | 2.9–14.6 | <0.0001 | 3.3% | 8.3 | 2.8–24.3 | 0.0001 | ||
| ≥65 | 3,233 | |||||||||
| VT−/ischemia− | 4.4% | Referent | 1.6% | Referent | ||||||
| VT+/ischemia− | 7.2% | 1.7 | 1.1–2.5 | 0.0083 | 3.2% | 1.8 | 1.0–3.4 | 0.05 | ||
| VT−/ischemia+ | 10.9% | 2.2 | 1.5–3.0 | <0.0001 | 3.5% | 2.1 | 1.2–3.7 | 0.01 | ||
| VT+/ischemia+ | 20.5% | 4.9 | 3.3–7.3 | <0.0001 | 10.9% | 5.4 | 2.9–10.0 | <0.0001 | ||
| Gender | ||||||||||
| Female | 2,221 | |||||||||
| VT−/ischemia− | 3.5% | Referent | 1.1% | Referent | ||||||
| VT+/ischemia− | 6.0% | 1.7 | 1.0–2.9 | 0.0489 | 2.1% | 1.8 | 0.7–4.4 | 0.19 | ||
| VT−/ischemia+ | 7.8% | 1.6 | 1.0–2.6 | 0.05 | 2.8% | 1.8 | 0.8–4.0 | 0.12 | ||
| VT+/ischemia+ | 15.8% | 4.2 | 2.4–7.5 | <0.0001 | 7.1% | 4.4 | 1.7–11.5 | 0.002 | ||
| Male | 4,124 | |||||||||
| VT−/ischemia− | 2.0% | Referent | 0.8% | Referent | ||||||
| VT+/ischemia− | 4.9% | 2.6 | 1.7–3.9 | <0.0001 | 2.6% | 2.6 | 1.4–4.8 | 0.003 | ||
| VT−/ischemia+ | 7.5% | 3.2 | 2.1–4.9 | <0.0001 | 2.6% | 2.9 | 1.5–5.6 | 0.001 | ||
| VT/+ischemia+ | 14.3% | 6.6 | 4.1–10.5 | <0.0001 | 8.2% | 8.0 | 4.1–15.6 | <0.0001 | ||
| Previous heart failure | ||||||||||
| No | 5,277 | |||||||||
| VT−/ischemia− | 2.0% | Referent | 0.8% | Referent | ||||||
| VT+/ischemia− | 3.6% | 2.0 | 1.3–3.0 | 0.0012 | 1.5% | 1.8 | 0.9–3.6 | 0.07 | ||
| VT−/ischemia+ | 5.2% | 2.5 | 1.7–3.8 | <0.0001 | 2.0% | 2.8 | 1.5–5.2 | 0.002 | ||
| VT+/ischemia+ | 10.6% | 5.5 | 3.5–8.7 | <0.0001 | 4.0% | 4.8 | 2.2–10.2 | <0.0001 | ||
| Yes | 1,068 | |||||||||
| VT−/ischemia− | 5.7% | Referent | 1.7% | Referent | ||||||
| VT+/ischemia− | 13.3% | 2.4 | 1.4–4.0 | 0.0005 | 5.4% | 3.0 | 1.4–6.5 | 0.005 | ||
| VT−/ischemia+ | 16.3% | 2.1 | 1.3–3.4 | 0.0025 | 5.0% | 2.0 | 0.9–4.5 | 0.09 | ||
| VT+/ischemia+ | 32.5% | 5.2 | 2.9–9.3 | <0.0001 | 24.2% | 9.8 | 4.5–21.6 | <0.0001 | ||
| Diabetes | ||||||||||
| No | 4,195 | |||||||||
| VT−/ischemia− | 1.9% | Referent | 0.7% | Referent | ||||||
| VT+/ischemia− | 4.8% | 3.0 | 2.0–4.6 | <0.0001 | 2.4% | 3.5 | 1.9–6.5 | <0.0001 | ||
| VT−/ischemia+ | 7.3% | 3.0 | 2.0–4.6 | <0.0001 | 2.5% | 2.8 | 1.4–5.3 | 0.003 | ||
| VT/+ischemia+ | 13.4% | 6.8 | 4.3–10.9 | <0.0001 | 7.4% | 7.8 | 3.9–15.5 | <0.0001 | ||
| Yes | 2,150 | |||||||||
| VT−/ischemia− | 3.8% | Referent | 1.2% | Referent | ||||||
| VT+/ischemia− | 6.1% | 1.5 | 0.9–2.5 | 0.1421 | 1.5% | 1.0 | 0.4–2.6 | 0.92 | ||
| VT−/ischemia+ | 8.4% | 1.9 | 1.2–3.1 | 0.0109 | 3.0% | 2.2 | 1.0–4.8 | 0.05 | ||
| VT+/ischemia+ | 18.3% | 4.6 | 2.6–8.3 | <0.0001 | 9.9% | 5.9 | 2.4–14.8 | 0.0001 | ||
| TIMI risk score | ||||||||||
| Low | 1,702 | |||||||||
| VT−/ischemia− | 7.3% | Referent | 3.6% | Referent | ||||||
| VT+/ischemia− | 3.5% | 4.9 | 2.1–11.4 | 0.0002 | 1.2% | 4.7 | 1.4–15.6 | 0.01 | ||
| VT−/ischemia+ | 1.2% | 1.8 | 0.5–6.6 | 0.36 | 0.6% | 2.8 | 0.5–14.5 | 0.23 | ||
| VT+/ischemia+ | 2.1% | 1.9 | 0.2–14.8 | 0.55 | 0.0% | 0.0 | 0.0– | 0.99 | ||
| Moderate | 3,336 | |||||||||
| VT−/ischemia− | 2.5% | Referent | 1.1% | Referent | ||||||
| VT+/ischemia− | 5.1% | 2.2 | 1.4–3.4 | 0.0007 | 2.3% | 2.0 | 1.1–3.9 | 0.03 | ||
| VT−/ischemia+ | 7.1% | 2.4 | 1.6–3.8 | <0.0001 | 2.5% | 1.8 | 0.9–3.6 | 0.1 | ||
| VT+/ischemia+ | 10.3% | 4.8 | 2.8–8.5 | <0.0001 | 3.5% | 4.0 | 1.7–9.3 | 0.002 | ||
| High | 1,307 | |||||||||
| VT−/ischemia− | 5.8% | Referent | 1.5% | Referent | ||||||
| VT+/ischemia− | 8.3% | 1.5 | 0.9–2.6 | 0.16 | 3.4% | 2.0 | 0.7–5.5 | 0.2 | ||
| VT−/ischemia+ | 12.5% | 2.3 | 1.4–3.7 | 0.0006 | 4.3% | 3.5 | 1.5–8.2 | 0.004 | ||
| VT+/ischemia+ | 25.1% | 5.8 | 3.5–9.6 | <0.0001 | 16.4% | 11.0 | 4.8–25.5 | <0.0001 | ||
| BNP (pg/ml) | ||||||||||
| ≤80 | 2,537 | |||||||||
| VT−/ischemia− | 0.8% | Referent | 0.3% | Referent | ||||||
| VT+/ischemia− | 2.7% | 2.8 | 1.2–6.4 | 0.0171 | 1.2% | 3.8 | 1.2–12.2 | 0.02 | ||
| VT−/ischemia+ | 5.7% | 4.8 | 2.4–9.3 | <0.0001 | 2.4% | 4.4 | 1.6–12.2 | 0 | ||
| VT+/ischemia+ | 11.1% | 11.4 | 4.9–26.5 | <0.0001 | 7.3% | 15.1 | 4.6–49.6 | <0.001 | ||
| >80 | 1,874 | |||||||||
| VT−/ischemia− | 5.4% | Referent | 1.7% | Referent | ||||||
| VT+/ischemia− | 8.1% | 1.5 | 1.0–2.3 | 0.0817 | 3.8% | 1.8 | 0.9–3.7 | 0.1 | ||
| VT−/ischemia+ | 11.1% | 1.6 | 1.1–2.5 | 0.0224 | 3.3% | 1.8 | 0.9–3.7 | 0.1 | ||
| VT/+ischemia+ | 19.7% | 3.5 | 2.2–5.7 | <0.0001 | 9.9% | 4.7 | 2.2–9.9 | <0.0001 | ||
| Troponin | ||||||||||
| Negative | 1,546 | |||||||||
| VT−/ischemia− | 0.9% | Referent | 0.6% | Referent | ||||||
| VT+/ischemia− | 3.4% | 2.6 | 1.0–7.0 | 0.0625 | 1.7% | 2.4 | 0.6–9.5 | 0.2024 | ||
| VT-/Ischemia+ | 4.1% | 3.3 | 1.4–8.1 | 0.0089 | 2.2% | 3.4 | 1.1–10.3 | 0.0325 | ||
| VT/+ischemia+ | 15.8% | 14.1 | 5.6–35.6 | <0.0001 | 10.5 | 15.3 | 4.7–49.9 | <0.0001 | ||
| Positive | 2,840 | |||||||||
| VT−/ischemia− | 3.6% | Referent | 1.0% | Referent | ||||||
| VT+/ischemia− | 6.0% | 1.6 | 1.0–2.4 | 0.0385 | 2.8% | 2.3 | 1.2–4.6 | 0.0167 | ||
| VT−/ischemia+ | 10.3% | 2.0 | 1.4–3.0 | 0.0005 | 3.2% | 2.2 | 1.1–4.4 | 0.0191 | ||
| VT+/ischemia+ | 16.7% | 4.2 | 2.7–6.7 | <0.0001 | 8.8% | 5.7 | 4.7–12.0 | <0.0001 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


