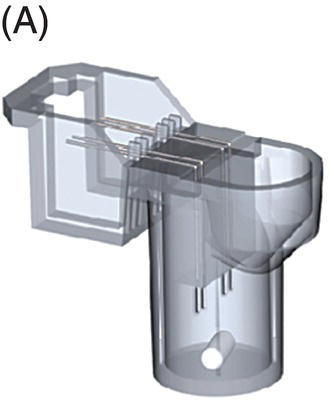
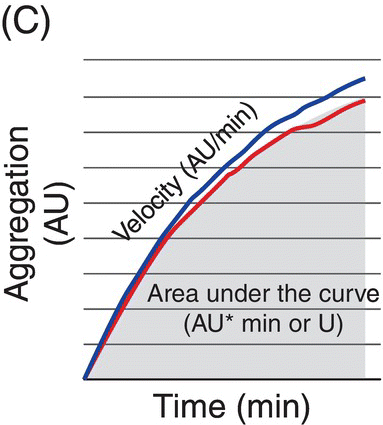
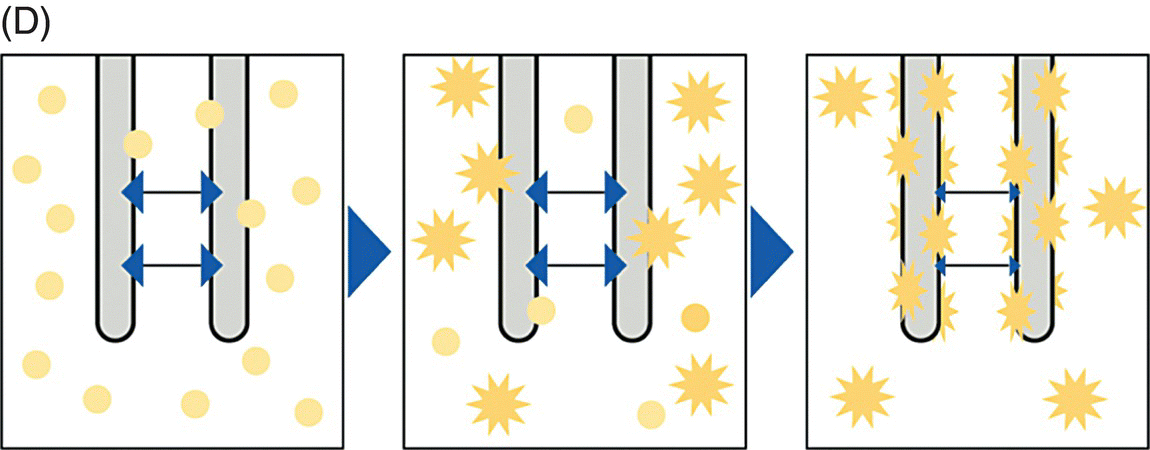
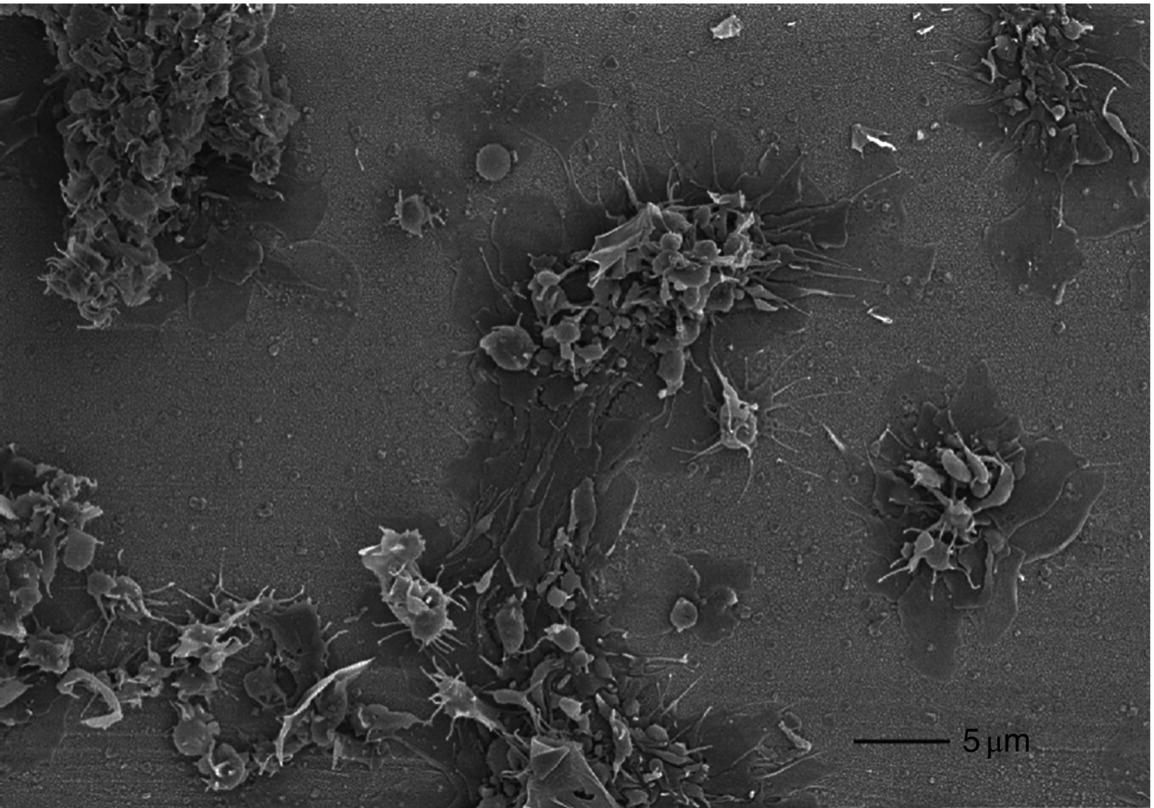
10 Martin Orban and Dirk Sibbing Klinikum der Universität München, Ludwig-Maximilians-Universität München, Munich, Germany Light transmission aggregometry (LTA), first described by Born in 1962 [1], was the established gold standard of platelet function measurement for decades. This method requires translucent suspensions like platelet-rich plasma (PRP) and measures platelet function in an artificial milieu. Besides optical aggregometry, another principle to assess platelet aggregation in whole blood and using electrical impedance was first described in 1979 by Cardinal and Flower [2]. The original method, however, lacks standardization, is relatively complicated, needs skilled personnel, and is therefore labor and time consuming – features that make them impractical to be incorporated into everyday clinical routine. To overcome the shortcomings of the latter – laboratory-based – methods, the Multiplate® analyzer (Verum Diagnostica GmbH, Roche, Germany) was developed in the last decade and was introduced in clinical practice. The Multiplate® analyzer is a benchtop multiple electrode impedance aggregometer (MEA) (Figure 10.1B), which measures changes in electrical impedance over time. The name Multiplate arises from the multiplicity of channels and sensors per channel of the device. One Multiplate® test cell incorporates two independent sensor units that serve as internal control at each test. One unit consists of two silver-coated highly conductive copper wires with a length of 3.2 mm (Figure 10.1A). After dilution (1:2 with 0.9% NaCl solution) of anticoagulated whole blood (hirudin is recommended for anticoagulation) and stirring for 3 min in the test cuvettes at 37°C, the testing reagents are added and aggregation is continuously recorded for a defined time span. During stimulation, platelets adhere to the metal surface of the electrodes, get activated, and aggregate (see Figure 10.1D and Figure 10.2). The increase of impedance due to the attachment of platelets to the electrodes is detected for each sensor unit separately during 6 min and transformed to aggregation units (AU) that are plotted against time (see Figure 10.1C). Approximately 8 AU correspond to 1 Ω. Aggregation measured with MEA is quantified as AU and area under the curve (AUC) of AU (AU*min). Electrical detection of impedance is calibrated by the manufacturer during production, and liquid controls are used to ensure quality control of impedance detection on-site. Figure 10.1 The Multiplate analyzer. The figure shows the components and principles of the electrical impedance aggregometer Multiplate® analyzer. (A) shows a Multiplate® test cell with two independent sensor units consisting of two pairs of electrodes, (B) shows the whole Multiplate® benchtop device, (C) shows the output of the device for platelet aggregation measurements that are plotted against time (in AU*min), and (D) shows an example of platelets clotting on the metal surface of the two electrodes of one sensor unit. By doing so, electrical impedance is rising between the two electrodes, and less current is flowing between the electrodes (arrows). (Source: Images kindly provided by Verum Diagnostica, Munich, Germany.) Figure 10.2 Electron microscopic imaging of a disposable Multiplate® analyzer test. Electron microscopic imaging (2000× magnified) of a disposable Multiplate® analyzer test cell showing platelets sticking on the surface of the electrode after a standard test. (Source: Image was kindly provided by Prof. Dr. Armin Reininger.) Currently, there are five certified testing stimuli available, which are (i) ASPI test (arachidonic acid) for the effect of aspirin, (ii) ADP test (adenosine diphosphate) for P2Y12 ADP antagonists, (iii) TRAP test (thrombin receptor-activating peptide-6) for GPIIb/IIIa inhibitors, (iv) COL test (collagen) for aspirin and GPIIb/IIIa inhibitors, and (v) RISTO test (ristocetin) for the detection of severe von Willebrand disease and Bernard–Soulier syndrome. The Multiplate® analyzer resembles a near-patient, semiautomated device and is well standardized, easy, and reliable to use, and the results can be available within 10 min after starting the testing process [3]. By using whole blood, which displays the natural environment of all blood components, it does not miss complex cellular interactions that can influence platelet function. The principle of platelet aggregation on a metal surface is likely to resemble the physiological process after plaque rupture or percutaneous coronary intervention (PCI). The method correlates well with the gold standard LTA [4] and is able to detect the effect of the commonly and most widely used antiplatelet agents, which are (i) the cyclooxygenase-1 inhibitor aspirin; (ii) the ADP receptor antagonists clopidogrel, prasugrel, and ticagrelor; and (iii) GPIIb/IIIa inhibitors. As the Multiplate® analyzer is a semiautomated device – in contrast to real point-of-care systems like the VerifyNow® system – it still requires sample preparation and pipetting. In addition, measurements are dependent on the hematocrit level and platelet count such that extreme values of these parameters may result in an imprecise assessment of platelet function. Similar as for other methods like LTA and the VerifyNow® system, the ADP test cannot be used to quantify P2Y12 receptor-directed platelet inhibition if GPIIb/IIIa inhibitors were administered before blood sampling. Different antagonists of the P2Y12 ADP receptor are in focus of contemporary research and clinical use of antiplatelet therapy monitoring because they are widely and extensively used; a status of high on-P2Y12 inhibitor treatment platelet reactivity (HPR) has been detected and is a known risk factor for ischemic events after PCI in elective patients and patients suffering from an acute coronary syndrome (ACS) [5]. The first studies that showed that higher platelet reactivity on clopidogrel treatment in patients undergoing PCI was linked to an elevated frequency of ischemic events in the past used predominantly the laboratory-based LTA [6, 7, 8]. Selected studies in which platelet reactivity was measured using the Multiplate® analyzer are presented in the following section (see Table 10.1). Toth et al. were among the first who performed a comparison between MEA assessed with the Multiplate® analyzer and single platelet counting (SPC) to measure platelet aggregation and inhibition by aspirin and apyrase in whole blood [9]. Platelet inhibition by apyrase and aspirin was comparable between MEA and SPC. The study also showed that the use of hirudin as an anticoagulant for MEA is preferable to the use of citrate. In 2009, we compared the ADP-induced platelet aggregation assessed with MEA and LTA in 149 patients undergoing PCI after clopidogrel loading with 600 mg. We were able to show a significant correlation (Spearman rank correlation coefficient = 0.71, p < 0.0001) between both methods. In a large prospectively designed study, we assessed the correlation between platelet reactivity measured with the Multiplate® analyzer after a high clopidogrel loading dose and the frequency of stent thrombosis (ST) in 1608 consecutive patients undergoing PCI. Clopidogrel low responders were defined as the upper quintile of platelet reactivity. Patients who showed a low response to clopidogrel had a significantly higher risk of suffering from an ST within 30 days after PCI compared to normal responders (2.2% vs. 0.2%; OR, 9.4; 95% CI, 3.1–28.4; p < 0.0001). The optimal cutoff value to predict an increased risk of the event of ST according to ROC analysis was ≥468 AU*min. The sensitivity and specificity of this cutoff value were 70% and 84%, respectively [10]. Siller-Matula and coworkers also found a correlation between HPR and ST in 416 patients undergoing PCI [11]. In their work, they could show that HPR detected by the Multiplate® analyzer can distinguish better between patients with or without ST compared to the laboratory-based VASP assay (area under the ROC curve, 0.92 for the Multiplate® analyzer, p
Multiplate Analyzer
Historical background
Principles of the Multiplate® analyzer

Advantages
Disadvantages
Clinical studies in cardiovascular medicine
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


