Multidetector row computed tomographic (MDCT) assessment of aortic annulus dimensions and frame position and deployment have been associated with paravalvular aortic regurgitation (PAVR) after transcatheter aortic valve implantation (TAVI). The present evaluation investigated the (pre- and postprocedure) MDCT associates of PAVR ≥2+. In total, 123 patients referred for TAVI underwent clinical evaluation, transthoracic echocardiography, and pre- and post-TAVI MDCT. Pre-TAVI MDCT measurements of the aortic annular dimensions and post-TAVI MDCT evaluation of the position and deployment of the prosthesis in the native annulus were performed. At 1-month follow-up, PAVR ≥2+ was observed in 25 patients (20%). The difference between the MDCT-derived maximum aortic annulus and the nominal diameters of the implanted prosthesis (odds ratio 1.912, p = 0.002) and shallow position of the frame in the left ventricular outflow tract (<2 mm) (odds ratio 4.865, p = 0.017) were independently related to significant PAVR. A maximum annulus diameter ≥2 mm larger than the nominal frame diameter had 72% sensitivity and 61% specificity to predict PAVR. In conclusion, in patients undergoing TAVI, ≥2-mm difference between maximum aortic annulus and nominal prosthesis diameters and depth of the frame into the left ventricular outflow tract of <2 mm are independently associated with PAVR ≥2+.
Transcatheter aortic valve implantation (TAVI) is an established alternative for patients with severe aortic stenosis and high operative risk mortality or contraindications for surgical aortic valve replacement. Paravalvular aortic regurgitation (PAVR) remains still as one of the main concerns of this therapy because the prognostic implications of PAVR are not negligible and data from the Placement of Aortic Transcatheter Valves trial cohort B have shown a twofold increased mortality among patients with mild or more PAVR compared with those showing none or trace PAVR. Determinants of PAVR are still debated. Of particular importance is the measurement of aortic valve annular dimensions with 3-dimensional imaging techniques such as multidetector row computed tomography (MDCT) because relative undersizing of the transcatheter prosthesis has been related to increased incidence of PAVR. In addition, position and deployment of the prosthesis have been suggested as relevant underlying mechanisms of PAVR after TAVI. However, few studies have demonstrated the relevance of postprocedural MDCT to identify the determinants of PAVR after TAVI. Accordingly, the present study aimed to identify the MDCT-derived pre- and postprocedural parameters independently associated with significant PAVR after TAVI.
Methods
Patients with symptomatic severe aortic stenosis and high risk or contraindications for surgical aortic valve replacement were evaluated for TAVI. A heart team consisting of clinical, imaging, and interventional cardiologists, cardiac surgeons, and anesthesiologists agreed on the indication for TAVI following the European Society of Cardiology and the European Association of Cardio-Thoracic Surgery guidelines. Before TAVI, all patients underwent clinical evaluation, including estimation of the operative risk based on logistic EuroSCORE, comprehensive thansthoracic echocardiography (TTE) to estimate the aortic stenosis severity, MDCT to size the aortic annulus and evaluate the anatomy and dimensions of the peripheral arteries, and invasive coronary angiography to exclude significant coronary artery disease amenable to percutaneous intervention. One month after TAVI, TTE was used to assess the prosthetic valve function, whereas the deployment and position of the prosthetic valve were evaluated on MDCT images. The pre- and postprocedural MDCT data on aortic root anatomy and geometry and apposition of the prosthetic valve within the native aortic annulus were related to the presence of significant PAVR after TAVI. Clinical, echocardiographic, and MDCT data were prospectively collected in an electronic clinical patient file (EPD vision version 8.3.3.6; Leiden, The Netherlands) and retrospectively analyzed. The Institutional Review Board approved this retrospective analysis.
TAVI was performed at the hybrid operating room under general anesthesia. Fluoroscopy was the mainstay imaging technique to guide the procedure assisted by transesophageal echocardiography (iE33; Philips Medical System, Andover, Massachusetts). A 23-, 26-, or 29-mm Edwards SAPIEN and SAPIEN XT valves (Edwards Lifesciences, Irvine, California) were implanted based on the dimensions of the aortic annulus. The transfemoral approach was the preferred delivery technique, whereas the transapical approach was performed in patients with nonsuitable peripheral artery anatomy or in patients in whom a 29-mm valve was implanted. During rapid right ventricular pacing, aortic valve balloon dilatation was performed and subsequently the balloon-expandable prosthesis valve was deployed. The presence of significant PAVR was evaluated with transesophageal echocardiography and reballooning of the prosthesis or valve-in-valve was performed as a bailout technique to reduce aortic regurgitation severity. Patients who underwent a valve-in-valve procedure were excluded from further analysis.
A commercially available ultrasound system (Vivid 7, E9; General Electric, Horten, Norway) was used for pre- and post-TAVI TTE. The preprocedural evaluation included the assessment of the valve morphology at the parasternal short-axis view, and the left ventricular outflow tract (LVOT) diameter was measured at the parasternal long-axis view. The peak and mean transaortic pressure gradients were assessed in the apical long-axis or 5-chamber views, and the aortic valve area was calculated with the continuity equation. Aortic stenosis was considered severe if aortic valve area was <1.0 cm 2 and/or the transaortic mean gradient was ≥40 mm Hg. Left ventricular end-diastolic and end-systolic volumes were calculated with the Simpson’s method and left ventricular ejection fraction was derived.
To evaluate the presence of PAVR after TAVI, color flow Doppler echocardiography was performed after optimization of Nyquist limit and gain settings. PAVR was evaluated on multiple echocardiographic views and conventional criteria such as the vena contracta width, the ratio of the regurgitant jet width to the LVOT diameter, pressure halftime, and the proportion of the circumference of the sewing ring occupied by the regurgitant jet were used to estimate the PAVR (0 absent, 1+ trace or mild, 2+ mild to moderate, 3+ moderate to severe, and 4+ severe). PAVR ≥2+ at the first postoperative month was considered significant.
All patients underwent pre- and post-TAVI MDCT of the aortic root using either a 64- or a 320-detector row computed tomography scanner (Aquilion 64; Toshiba Medical Systems, Otawara, Japan and Aquilion ONE; Toshiba Medical Systems, Tochigi-ken, Japan). With the Aquilion 64 system, the data were acquired with a collimation of 64 × 0.5 mm and a gantry rotation time of 400 ms, whereas the tube current was 300 to 400 mA and the voltage was 120 or 135 kV, depending on body mass index of the patients. With the Aquilion ONE system, the data were acquired with a collimation of 320 × 0.5 mm, gantry rotation time of 350 ms, and tube current and voltage set at 400 to 580 mA and 100, 120, or 135 kV (based on body mass index of the patients), respectively. Unless contraindicated, patients received β blockers if their heart rate was ≥70 beats/min. All scans were performed during midinspiratory breath hold, and 80 to 90 ml of nonionic contrast (Iomeron 400; Bracco, Milan, Italy) was injected into the antecubital vein. Subsequently, data sets were reconstructed and off-line postprocessing of MDCT images was performed on dedicated workstations (Vitrea 2; Vital Images, Minneapolis, Minnesota).
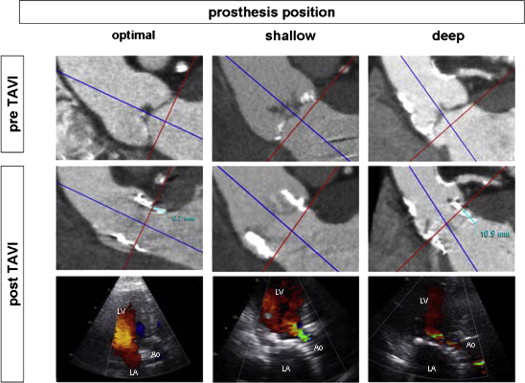
Diastolic and systolic images of the aortic root at the respective 75% and 30% to 40% of RR interval were selected. By aligning the 3 orthogonal multiplanar reformation planes, the double-oblique transversal plane that bisects the aortic annulus beneath the hinge points of the aortic cusps was obtained. At this level, the minimum and maximum aortic annulus diameters and the annulus area were measured. From the orthogonal sagittal and coronal views, the aortic annulus diameters were also measured as previously described. In addition, from the non–contrast enhanced images, the calcium Agatston score of the aortic valve and landing zone was calculated. On 1-month follow-up MDCT scans, the prosthesis deployment and position in relation to aortic root were evaluated. Particularly, the distance between the lower rim of the prosthesis frame in the LVOT and the native aortic annulus at the level of the left coronary cusp was measured ( Figure 1 ). Moreover, the distance between the upper rim of the valve frame and the right and left coronary ostia was evaluated. Additionally, the prosthesis deployment was visualized at the double-oblique transverse plane of the aortic annulus. At this level, the area of the deployed prosthetic valve was assessed by planimetry, and additionally, the maximal and minimal diameters of the prosthetic valve frame were measured. Following previous studies, an eccentricity index of, calculated as [1 − (minimum prosthesis diameter/maximum prosthesis diameter)], ≥0.1 defined a noncircular deployment of the prosthesis. Moreover, shallow or deep implantation of the frame were evaluated and defined as depth of the frame in the LVOT of <2 or >8 mm from the level of the hinge point of the left coronary cusp, respectively ( Figure 1 ). Additionally, the difference between the MDCT-derived coronal and maximal diameters of the aortic annulus and the nominal diameter of the implanted prosthesis were calculated. Furthermore, the difference between the MDCT-derived aortic annular and the nominal areas of the implanted prosthesis was also assessed. Among several pre- and post-TAVI MDCT parameters, the determinants of PAVR were evaluated.
All analyses were performed with a package of SPSS software version 17 (SPSS Inc., Chicago, Illinois). Based on visual inspection of the histograms and the Kolmogorov-Smirnov tests, continuous variables were considered normally distributed and are presented as mean and SD or nonnormally distributed and presented as median and interquartile range. Categorical variables are presented as number and frequencies. Patients were categorized according to the presence of nonsignificant PAVR (<2+) or significant PAVR (≥2+) at 1-month follow-up. Continuous variables were compared with the unpaired Student t test if normally distributed or the Mann-Whitney test otherwise. Categorical variables were also compared with the chi-square or Fisher’s exact test, as appropriate. Receiver operating characteristic curve analyses were performed to assess the accuracy of several MDCT parameters to predict the presence of PAVR ≥2+, and the cut-off values for each variable were obtained from the highest sum of sensitivity and specificity. Univariate and mutivariate logistic regression analyses were performed to evaluate independent determinants of PAVR ≥2+, and the estimated odds ratios and the 95% confidence intervals were calculated. Variables with a p <0.1 in the univariate analysis were included in the multivariate model. A 2-sided p <0.05 was considered statistically significant.
Results
A total of 123 patients (81 ± 7 years, 49% men) with symptomatic severe aortic stenosis treated with TAVI and complete evaluation including pre- and post-TAVI MDCT were evaluated. The baseline characteristics of the patients are listed in Table 1 . Repeated balloon dilatation of the prosthesis was performed in 15 patients (12%). In 10 patients (66%), PAVR significantly reduced (<2+) after this intervention.
| Variable | Overall, n = 123 (%) | PAVR <2+, n = 98 (%) | PAVR ≥2+, n = 25 (%) | p |
|---|---|---|---|---|
| Age (yrs) | 81 ± 7 | 81 ± 7 | 80 ± 7 | 0.446 |
| Men | 60 (49) | 50 (51) | 10 (40) | 0.325 |
| Body surface area (m 2 ) | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.4 | 0.998 |
| Hypertension | 47 (38) | 38 (39) | 9 (36) | 0.799 |
| Diabetes mellitus | 36 (29) | 30 (30) | 6 (24) | 0.517 |
| Peripheral vascular disease | 23 (19) | 21 (21) | 2 (8) | 0.158 |
| Smoking | 28 (22) | 23 (23) | 5 (20) | 0.796 |
| Coronary artery disease | 87 (71) | 70 (71) | 17 (68) | 0.737 |
| New York Heart Association functional classes III and IV | 75 (61) | 62 (63) | 13 (52) | 0.303 |
| Pacemaker | 12 (10) | 10 (10) | 2 (8) | 1.000 |
| Atrial fibrillation | 29 (24) | 21 (21) | 8 (32) | 0.266 |
| Medications | ||||
| β blockers | 71 (58) | 56 (57) | 15 (60) | 0.796 |
| Diuretics | 76 (62) | 59 (60) | 17 (68) | 0.474 |
| Statins | 74 (60) | 62 (63) | 12 (48) | 0.164 |
| Calcium antagonists | 36 (29) | 29 (29) | 7 (28) | 0.876 |
| Logistic EuroSCORE | 23.4 ± 14.1 | 23.4 ± 14.3 | 21.6 ± 13.6 | 0.567 |
| Aortic valve area (cm 2 ) | 0.73 ± 0.18 | 0.72 ± 19 | 0.78 ± 0.15 | 0.108 |
| Mean transaortic valve gradient (mm Hg) | 42 ± 15 | 42 ± 15 | 39 ± 13 | 0.421 |
| Left ventricular end-systolic volume (ml) | 60 ± 40 | 60 ± 40 | 62 ± 39 | 0.771 |
| Left ventricular end-diastolic volume (ml) | 112 ± 48 | 113 ± 48 | 109 ± 47 | 0.733 |
| Left ventricular ejection fraction (%) | 50 ± 13 | 50 ± 13 | 48 ± 13 | 0.510 |
| TAVI approach | 0.799 | |||
| Transfemoral | 47 (38) | 38 (36) | 9 (32) | |
| Transapical | 76 (62) | 60 (64) | 16 (68) | |
| Edwards SAPIEN valve (mm) | 0.088 | |||
| 23 | 29 (24) | 19 (22) | 10 (40) | |
| 26 | 93 (75) | 78 (76) | 15 (60) | |
| 29 | 1 (1) | 1 (2) | 0 (0) |
At 1-month follow-up, all patients underwent TTE. In 30 patients (24%), no PAVR was observed, whereas in respective 68 (55%), 22 (18%), and 3 (2%) patients, trivial-to-mild, mild-to-moderate, and moderate-to-severe PAVR were documented. Therefore, significant PAVR ≥2+ was observed in 25 patients (20%). In 5 of these patients, a repeat balloon dilatation was performed.
Patients with PAVR <2+ and ≥2+ had comparable Agatston aortic valve scores and similar aortic valve annular diameters and area measured at preprocedural MDCT scans ( Table 2 ). However, the difference between the aortic annulus diameter measured in the coronal plane and the nominal diameter of the implanted prosthesis was significantly larger in patients with PAVR ≥2+ compared with those without significant PAVR. Similarly, the difference between the maximum aortic annulus diameter measured at the double-oblique transverse cross-sectional plane and the nominal diameter of the implanted prosthesis was significantly larger in patients with PAVR ≥2+ compared with those without significant PAVR. In contrast, there were no significant differences between groups in terms of difference between aortic annular area and nominal frame area ( Table 2 ).
| Variable | PAVR <2+ (n = 98) | PAVR ≥2+ (n = 25) | p |
|---|---|---|---|
| Agatston aortic valve score | 2,612 ± 1,394 | 2,666 ± 1,222 | 0.862 |
| Aortic annular dimensions | |||
| Coronal diameter (mm) | 25.4 ± 2.1 | 26.0 ± 2.5 | 0.271 |
| Sagittal diameter (mm) | 22.8 ± 2.0 | 22.6 ± 2.2 | 0.551 |
| Maximum diameter (mm) | 27.1 ± 2.4 | 27.9 ± 2.3 | 0.092 |
| Minimum diameter (mm) | 21.1 ± 2.0 | 21.2 ± 1.9 | 0.868 |
| Planimetered area (cm 2 ) | 4.3 ± 0.8 | 4.2 ± 0.8 | 0.694 |
| Difference between coronal diameter of the aortic annulus and the nominal diameter of the implanted prosthesis (mm) | 0.03 ± 1.8 | 1.23 ± 1.8 | 0.004 |
| Difference between maximum diameter of the aortic annulus and the nominal diameter of the implanted prosthesis (mm) | 1.6 ± 2.2 | 3.1 ± 1.6 | 0.004 |
| Difference between the aortic annulus area and the nominal frame area (cm 2 ) | −0.8 ± 0.7 | −0.7 ± 0.7 | 0.237 |
| Prosthetic valve eccentricity of ≥0.1 (%) | 10 (10) | 6 (24) | 0.067 |
| Depth of frame in the LVOT of <2 mm (%) | 7 (7) | 7 (28) | 0.003 |
| Depth of frame in the LVOT of >8 mm (%) | 7 (7) | 3 (12) | 0.428 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


