Chapter 15 Morbid Obesity
Obesity: Magnitude of the Problem
Morbid obesity is defined as being 100 lb above ideal body weight, twice ideal body weight, or a body mass index (BMI, which is weight [in kg]/height [in m2]) of 40 kg/m2. The latter definition is more accepted internationally and has essentially replaced the former ones for all practical and scientific purposes. A consensus conference by the National Institutes of Health (NIH) in 1991 suggested that the term severe obesity is more appropriate for defining people of this size.1 This term shall be used interchangeably with morbid obesity in the remainder of this chapter.
It is estimated that more than one third of the U.S. adult population is obese; the prevalence of morbidly obese adults with a BMI of 40 or higher has gone from 2.9% in 1994 to 5.9% of the adult U.S. population in the National Health and Nutrition Examination Survey (NHANES) in 2006.2 Patients undergoing bariatric surgery in the United States have average BMIs that are significantly higher than those reported in Europe. Australia, however, is not far behind, according to Australian bariatric surgeons. Even Europe, where severely obese individuals are not common in crowds, is now experiencing an overall enlargement of its population. Studies of adolescent obesity have estimated the incidence of obesity (40% above ideal body weight) as being in the 35% range for adolescents in the United States but more than 20% in most European countries. The problem is also growing at an alarmingly rapid rate in the United States. In 1985, when statistics of national obesity were first measured by the Centers for Disease Control and Prevention according to individual states, many states had no such data available. Of the approximately 50% that has these data, more than 50% reported a less than 10% incidence of people with a BMI higher than 30 kg/m2. By 2008, every state except Colorado had reported that the incidence had risen to higher than 20% and 6 states reported that more than 30% of their population had a BMI of 30.0%.
Obesity is estimated to cause 300,000 deaths annually in the United States, whereas the total number of deaths annually from breast and colon cancers is only approximately 90,000/year.3 After tobacco use, obesity is the second leading cause of preventable death in the United States and is second to smoking on the list of preventable factors responsible for increased health care costs. It is a sobering thought to realize that a 25-year-old morbidly obese man has a 22% reduction in life expectancy, or 12 years of life lost, when compared with a normal-sized man.3 There is speculation that within the next decade, obesity may overtake tobacco as the leading cause of preventable death in the United States.
Pathophysiology and Associated Medical Problems
The pathophysiology of severe obesity is poorly understood. Debate is ongoing regarding the relative genetic versus environmental components of the disease. There is a clear familial predisposition; it is rare for a single family member to have severe obesity and there is increasing evidence of specific genes, including FTO (fat mass and obesity-related) and MC4R (melanocortin 4 receptor) associated with obesity, increased fat mass, and insulin resistance.4,5 The rapid increase in obesity from 1980 to 2010 emphasizes the considerable influence of environmental factors, such as easily available, cheap, high-density, caloric-rich foods and physical inactivity promoted by widespread ownership of cars, which also contributes to the problem.
Another explanation of the obesity epidemic is that during human development, for thousands of years, the so-called thrifty gene marked those who could survive the periods of extreme protein calorie deprivation that marked early human development.6 Because this thrifty gene allowed more efficient absorption and use of the calories ingested, the humans who had the thrifty gene had a distinct survival or fertility advantage. However, in modern society, in which we can drive through a fast food restaurant, drink high-fructose corn syrup soda, and eat a fat-laden, super-sized hamburger without getting out of the car, the thrifty gene does not convey a survival advantage. Instead, it helps increase the intake of calories in excess of metabolic needs.
We know that hormones, peptides, and vagal afferents to the brain have a major influence on satiety, appetite, and energy intake. The appetite hormone ghrelin, produced largely in the proximal part of the stomach by the presence of food, is involved in appetite and satiety.7 Increased levels of ghrelin lead to increased food intake and increased ghrelin levels develop in individuals who are on low-calorie diets. This suggests one possible mechanism for the failure of most diets after 6 months—the increase in ghrelin. Interestingly, patients have normal to elevated ghrelin levels after laparoscopic AGB. Most studies have suggested that patients undergoing gastric bypass have suppressed postoperative levels of ghrelin, and appetite is dramatically reduced after gastric bypass, which leads to the incredible decrease in caloric intake that also leads to massive weight loss in the first 12 to 18 months after RYGB.8
Morbid obesity is a metabolic disease associated with numerous medical problems, some of which are almost unknown in the absence of obesity (Box 15-1). These problems must be carefully considered when one is contemplating offering weight reduction surgery to a patient. The most frequent problem is the combination of arthritis and degenerative joint disease, present in at least 50% of patients seeking surgery for severe obesity. The incidence of sleep apnea is high. Asthma is present in more than 25%, hypertension in more than 30%, diabetes in more than 20%, and gastroesophageal reflux in 20% to 30% of patients. The incidence of these conditions increases with age and with the severity and duration of the severe obesity.
Box 15-1 Medical Conditions Associated With Severe Obesity
Metabolic
Metabolic syndrome (abdominal obesity, hypertension, dyslipidemia, insulin resistance)
Nonalcoholic steatotic hepatitis (NASH) or nonalcoholic fatty liver disease (NAFLD)
Not listed in Box 15-1 are the associated societal discriminatory problems faced by severely obese individuals. Public facilities in terms of seating, doorways, and restroom facilities often make access to events held in these settings unavailable to a severely obese person. Travel on public transportation is sometimes difficult, if not impossible, especially in regard to air travel. Employment discrimination clearly exists for these individuals. Finally, the combination of low self-esteem, frequently a history of sexual or physical abuse, and these social difficulties coalesce to create a high incidence of depression in the morbidly obese patient population.
Medical Versus Surgical Therapy
Medical therapy for severe obesity has limited short-term success and almost nonexistent long-term success. Once severely obese, the likelihood that a person will lose enough weight by dietary means alone and remain at a BMI below 35 kg/m2 is estimated at 3% or less. The NIH consensus conference recognized that for this patient population, medical therapy has been uniformly unsuccessful in treating the problem.1 A more recent review of the clinical trials of lifestyle interventions for the prevention of obesity in children has demonstrated that most were completely ineffective and the few that were marginally effective had an extremely small impact on BMI.9 Despite massive efforts health care providers to influence weight through diet, physical activity, and lifestyle changes, the only effective long-term method for weight loss has been shown to be bariatric surgery. In a head to head trial, O’Brien and colleagues10 randomized obese adolescents to lap band or to diet and lifestyle changes. Patients randomized to lap band lost 34.6 kg compared with the diet group, who lost 3.0 kg at the end of the 2-year trial. In another trial of obese adults, the surgical group achieved a 21.6% initial body weight loss whereas the medical group had a paltry 5.5% of initial body weight loss.11
The Swedish Obesity Study (SOS) is our best evidence of the profound salutary effects of bariatric surgery on morbidity and mortality.12 The study followed 98.9% of subjects undergoing bariatric surgery—gastric bypass, vertical banded gastroplasty, or nonadjustable silicone gastric banding—compared with a group of age-, gender-, and BMI-matched control subjects undergoing standard medical treatment. There was a significant long-term reduction in weight and in comorbid conditions, which resulted in a significant reduction in mortality in the bariatric surgery patients. Other excellent long-term studies have confirmed the benefits of bariatric surgery and have indicated a significant reduction in weight and long-term mortality in patients undergoing gastric bypass.13
Recently, experts from around the world met in Rome as part of the Diabetes Surgery Summit and put forth a position statement on recommendations for clinical and research issues related to the development of diabetes surgery.14 During this extraordinary meeting, medical and surgical experts on bariatric surgery, obesity, and type 2 diabetes crafted a statement to develop methods for diabetes surgery to help improve access to proven surgical options while also suggesting research avenues to be pursued. Some of the recommendations included investigation of surgery on patients who have a BMI lower than 35, the previously defined cutoff for weight loss surgery.
Preoperative Considerations
Evaluation and Selection
Eligibility
Selection of patients for bariatric surgery is based strictly on currently accepted NIH guidelines. Patients must have a BMI higher than 40 kg/m2 without associated comorbid medical conditions or a BMI higher than 35 kg/m2 with an associated comorbid medical problem. They must have also failed dietary therapy. Beyond this, the NIH guidelines are not specific. However, it has been my experience that several practical criteria must also be used as guidelines for indications for surgery, including psychiatric stability, motivated attitude, and the ability to comprehend the nature of the operation and resultant changes in eating behavior and lifestyle. Criteria for eligibility for bariatric surgery are given in Box 15-2. An inability to fulfill these criteria is a contraindication to bariatric surgery.
Box 15-2 Indications for Bariatric Surgery
Patients must meet the following criteria for consideration for bariatric surgery:
One criterion not listed in Box 15-2 that unfortunately is often a significant issue for a severely obese patient is insurance coverage for the operation. Although bariatric surgery has been one of the most commonly studied operative procedures, with abundant information from controlled studies showing a significant survival advantage to the patient undergoing surgery, many insurance companies refuse to cover the procedure or establish multiple barriers to coverage for the individual patient. The Centers for Medicare and Medicaid Services (CMS), the federal agency that sets Medicare guidelines, established criteria for the coverage of open and laparoscopic gastric bypass, laparoscopic AGB, and DS operations in 2006. A controversial aspect of the ruling is the requirement that bariatric surgery be performed only by surgeons in hospitals that are designated as Centers of Excellence by the American Society of Bariatric Surgeons or level I centers by the American College of Surgeons. These unique requirements for Medicare beneficiaries were at least partly the results of concern by policymakers that the morbidity and mortality associated with bariatric surgery were high and that the explosive growth in the number of hospitals and surgeons performing the procedures did not correlate with hospital oversight of these procedures and resulting complications. Nevertheless, this marks a watershed moment in surgery in which, increasingly, payers are demanding that surgeons and hospitals meet stringent requirements for infrastructure, training of personnel, and ultimately, results of the procedures. Bariatric surgeons have risen to the occasion, as evidenced by the most recent data from National Surgical Quality Improvement Program (NSQIP)15 and the Longitudinal Assessment of Bariatric Surgery (LABS) consortium16 of the extremely low morbidity and mortality for laparoscopic bariatric surgery across the United States. These recent studies suggest that the imposition of the Center of Excellence standard has been at least partially responsible of the decline in operative morbidity and mortality.
Age is a controversial contraindication to bariatric surgery. For adolescents, most pediatric bariatric surgeons recommend that the operation be performed after the major growth spurt (mid to late teens), thus allowing increased maturity on the part of the patient. Simple restrictive operations are thought to be most appropriate for patients in this age group. In the United States, although the laparoscopic AGB procedure (LAP-BAND) has been approved by the U.S. Food and Drug Administration (FDA) only for patients 18 years or older, several groups have modest experiences using this device under FDA guidelines.10 Increasing experience will be required to determine which operation is most effective in adolescents.
Evaluation
Preoperative assessment of a bariatric surgical patient involves two distinct areas. One is a specific preoperative assessment of candidacy for bariatric surgery and evaluation for comorbid conditions. The second is a general assessment and preoperative preparation, as for any major abdominal surgery, which is discussed in depth in Chapter 11.
General Measures
A team approach is required for the optimal care of a morbidly obese patient (Box 15-3). Box 15-4 summarizes the steps and tests routinely performed for the preoperative evaluation of bariatric patients in the my clinics.
Box 15-3 Bariatric Multidisciplinary Team
Operating room scrub technician, nurse
Nurse care coordinator, educator
Box 15-4 Preoperative Evaluation
Before the Clinic Visit
Documented, medically supervised diet
Counseling and referral from the primary care physician
Initial Clinic Visit
Group presentation on information in the booklet
Group presentation on preoperative and postoperative nutritional issues by the nutritionist
Individual assessment by the surgeon’s team
Individual counseling session with the surgeon
Individual counseling session with the nutritionist
Subsequent Events and Evaluations
Full psychological assessment and evaluation, as indicated
Medical specialist evaluations, as indicated
Insurance approval for coverage of the procedure
Screening flexible upper endoscopy, as indicated
Screening ultrasound of the gallbladder (if present)
A first-generation cephalosporin, in a dose appropriate for weight, is given preoperatively, and antibiotics are continued for less than 24 hours. Data support the use of preoperative antibiotics, but no data have established the optimal regimen for deep venous thrombosis (DVT) prophylaxis. In the era of open surgery, pulmonary embolism (PE) was one of the most common causes of death after bariatric surgery. However, recent data have shown that pulmonary embolism is uncommon after laparoscopic RYGB and that measures such as early ambulation and sequential compression devices, without pharmacologic agents, such as heparin, can be used successfully to prevent DVT and PE in many patients undergoing laparoscopic gastric bypass or laparoscopic AGB.17 High-risk patients (e.g., those with history of DVT, venous stasis ulcers, known or highly suspected pulmonary hypertension, hypoventilation syndrome of obesity, or need for reoperation during the initial hospitalization) are given SC injections of heparin or low-molecular-weight heparin (LMWH) on call in the operating room and then twice daily until discharge at home, for a full 2-week course. Prophylactic vena cava filters are inserted, if possible on a temporary basis, in patients at extremely high risk for DVT and PE.
Specific Comorbid Conditions
Pulmonary assessment includes a search for obstructive sleep apnea because a significant number of patients undergoing bariatric surgery will have undiagnosed obstructive sleep apnea.16 A history of falling asleep while driving or at work or a history of feeling tired after a night’s sleep, coupled with a history of snoring or even witnessed apnea, is strongly suggestive of the condition. Patients with suggestive histories of clinically significant sleep apnea need to undergo preoperative sleep study testing. If found to have the condition, use of a continuous or bilevel positive airway pressure apparatus postoperatively while sleeping can eliminate the stressful periods of hypoxia that would otherwise result in these patients. Although tolerated under normal circumstances, these hypoxic episodes in the immediate postoperative period are more dangerous because of the enhanced effect of narcotic pain medications and postoperative fluid shifts, which affect hemodynamic stability.
Operative Procedures
Primary laparoscopic bariatric operations are preferred over the open procedures because of the increased availability of the laparoscopic approach and overwhelming advantages of the laparoscopic approach. These include reduced mortality, wound infections, pulmonary complications, thromboembolic complications, reduced rate of incisional hernias, and decreased hospitalization.15,16
We are only just now starting to recognize the many reasons why bariatric operations produce weight loss. The primary pathway to weight loss is the profound and long-lasting reduction of oral intake induced by changes in the gut-brain axis.18 The other is malabsorption of ingested food. Box 15-5 lists the major procedures to be described.
Box 15-5 Bariatric Operations
Mechanism of Action
Restrictive
Vertical banded gastroplasty (VBG; historic purposes only)
Laparoscopic adjustable gastric banding (AGB)
Laparoscopic Adjustable Gastric Banding
The AGB procedure may be performed with any of various types of adjustable bands. The two bands approved for use by the FDA in the United States are the LAP-BAND (INAMED Health, Santa Barbara, Calif) and the Realize band (Ethicon Endo-Surgery, Cincinnati, Ohio). The Swedish Adjustable Gastric Band (Obtech Medical, Baar, Switzerland), the MIDBAND (Medical Innovation Development, Villeurbanne, France), and the Heliogast band (Helioscopie, Vienne, France) are other banding systems used in Europe, Asia, the Middle East, and South America. The techniques of placement of the bands are similar; only the locking mechanisms, band shape and configuration, and adjustment schedules vary somewhat for the different types of bands. They all work on the principle of reduction of oral intake by augmenting the early satiety and decrease in appetite triggered by distention of the proximal part of the stomach and feedback to the brain eating center via the vagal nerves.18 Their advantage over other bariatric procedures is adjustability and a markedly lower initial operative morbidity and mortality.
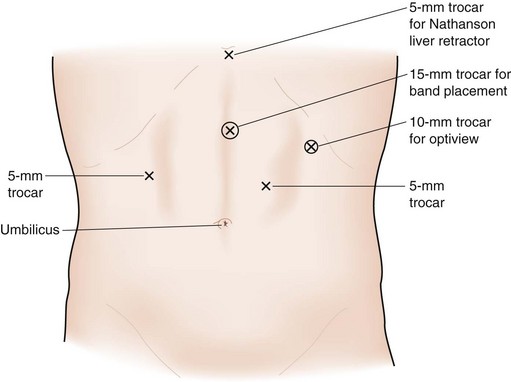
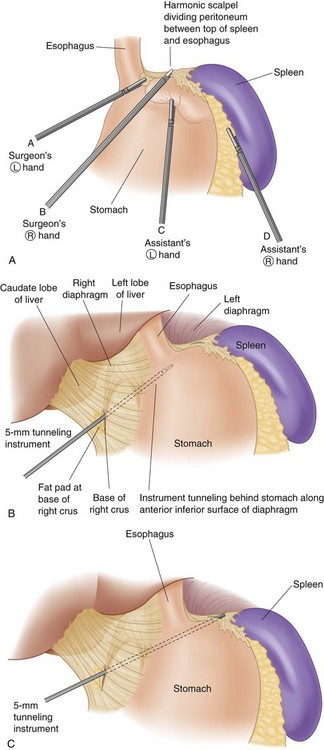
Trocar placement for AGB is shown in Figure 15-1. The surgeon stands to the patient’s right, the assistant is to the patient’s left, and the camera operator is adjacent to the surgeon. Most surgeons place the patient in the supine position, but some prefer to have the patient’s legs spread so that the surgeon can stand between his or her legs. The peritoneum at the angle of His is divided to create an opening in the peritoneum between the angle of His and the top of the spleen (Fig. 15-2A). The telescope is placed through the left upper quadrant port for this part of the operation to maximally view the angle of His area.
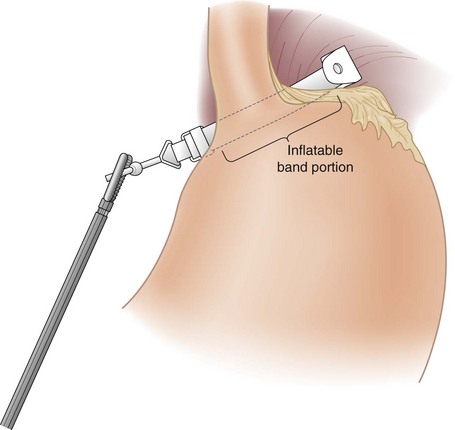
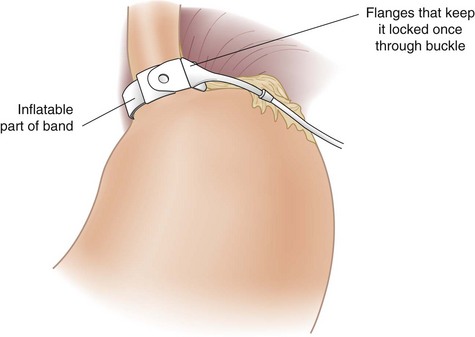
The pars flaccida technique has become the approach of choice for placing the adjustable band. It begins by dividing the gastrohepatic ligament in its thin area, just over the caudate lobe of the liver. The anterior branch of the vagus nerve is spared, and any aberrant left hepatic artery is preserved. The base of the right crus of the diaphragm is identified. Care must be taken to identify the crus clearly because occasionally the vena cava can lie close to the caudate lobe. The surgeon gently follows the surface of the right crus posterior and inferior to the esophagus while aiming for the angle of His (see Fig. 15-2B). A gentle spreading and pushing technique is used to create an avascular tunnel along this plane. Once the tip of the tunneling instrument is seen near the top of the spleen, it is gently pushed through any remaining peritoneal layers to complete the tunnel (see Fig. 15-2C). The adjustable band has already been placed in the peritoneal cavity through the large 15-mm trocar located in the right upper quadrant before dissection of the pars flaccida. The narrow end of the band itself is grasped by the tunneling instrument and pulled through the tunnel from the greater to the lesser side of the stomach (Fig. 15-3). That end is then threaded through the locking mechanism of the band, after which the band is locked. Once the band has been locked in place, the buckle is adjusted to lie on the lesser curvature side of the stomach (Fig. 15-4). A 5-mm grasper inserted between the band and stomach ensures that the band is not too tight.
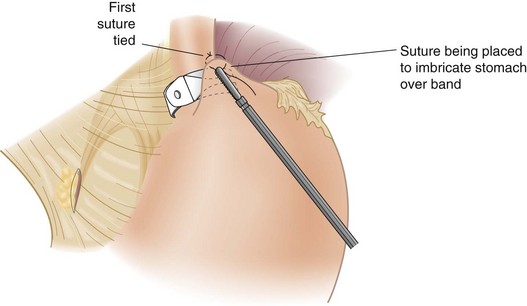
The anterior gastric wall is plicated over the band with three or four interrupted nonabsorbable sutures (Fig. 15-5). There needs to be just enough stomach above the level of the band for incorporating that tissue into the suture. Suturing is carried as far posterolaterally as possible because this region has been the most frequent area of fundus herniation through the band. The band is thus ideally secured approximately 1 cm below the gastroesophageal junction with this technique.
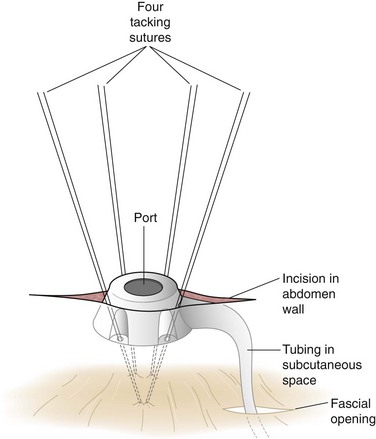
The Silastic tubing leading from the band is pulled through the 15-mm trocar site in the right upper quadrant paramedian area to complete the laparoscopic portion of the operation. The trocar site incision is enlarged to reveal the anterior rectus fascia, which is exposed approximately 2 to 4 cm lateral to the existing fascial defect for the trocar, and the access port is connected to the inflation tubing. Four sutures inserted through the four holes on the access port are placed in the fascia, after which the port is tied to the fascia (Fig. 15-6). The redundant tubing is replaced in the abdominal cavity, with care taken to avoid kinking.
Roux-en-Y Gastric Bypass
Described here is one technique that incorporates many of these modifications. There are certainly many variations of this technique and many, if not most, will yield excellent results. The essential principles of the operation are listed in Box 15-6.
Box 15-6 Essential Components of Roux-en-Y Gastric Bypass
Gastric pouch divided from the distal part of the stomach
Roux limb at least 75 cm in length
Enteroenterostomy constructed to avoid stenosis or obstruction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree