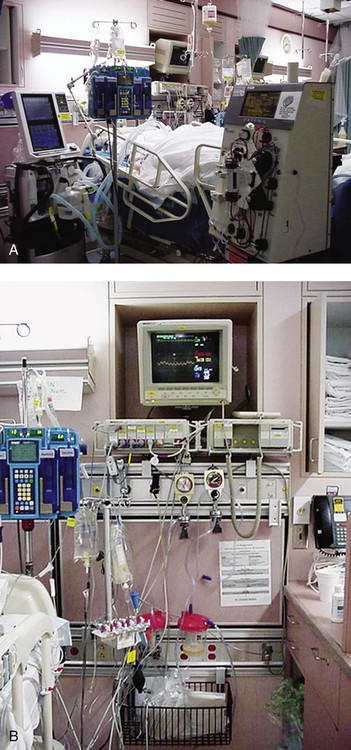
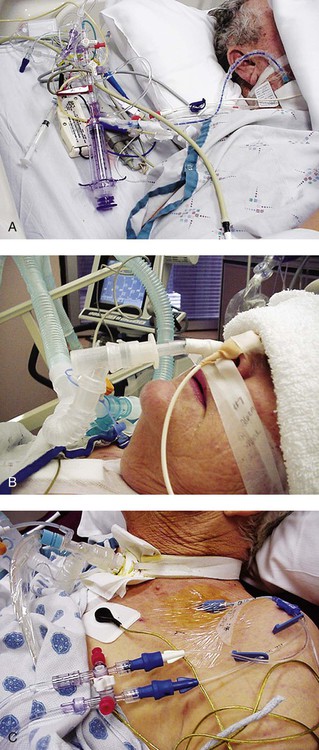
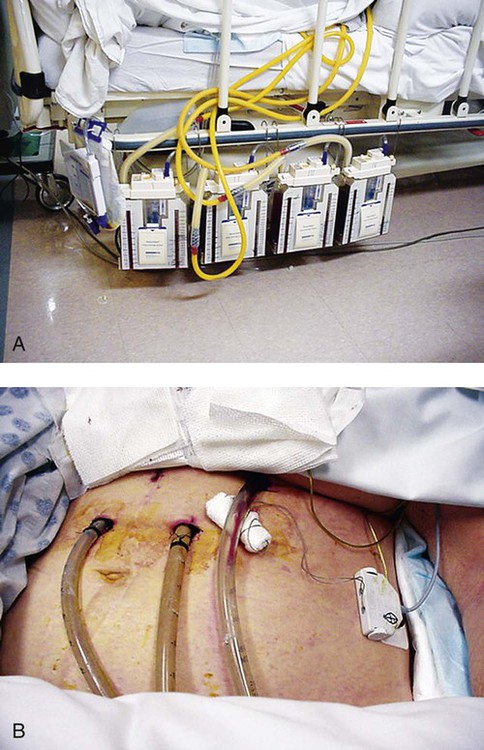
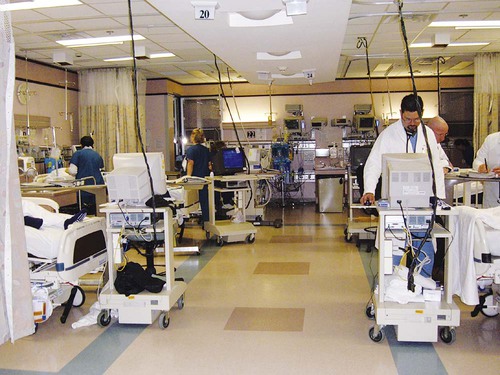
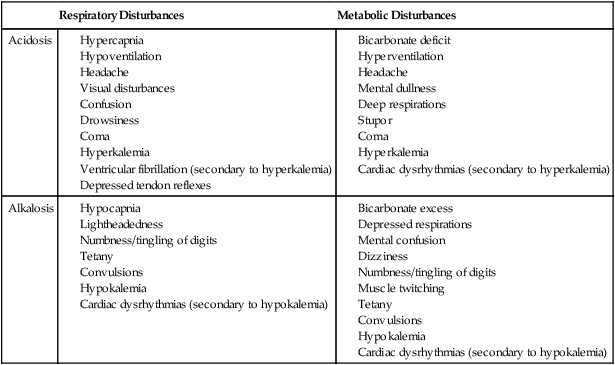
Familiarity with the extensive monitoring facilities in the ICU relieves some of the apprehensions the physical therapist may have about practicing in this setting. Such monitoring is central to the standard of physical therapy care in the ICU to guide progressive mobilization and management overall.1 On introduction to the unit, the physical therapist is immediately struck by the high-tech environment. Quality care in this setting depends on harnessing the potential of high-tech monitoring equipment to optimize assessment, evaluate treatment parameters, and establish anticipated and actual effectiveness of intervention, as well as to reduce untoward risk for the patient. Figure 16-1 illustrates a general view of a typical ICU. A view of a patient’s bedside area in the ICU shows life-support equipment and the various lines and catheters that are in place (Figure 16-2). A closer view of the patient demonstrates precisely where the various lines and catheters are positioned and identifies where caution must be observed. Treatments are modified according to the types and positions of the lines and catheters for each patient (Figure 16-3). Imbalances are reflected as excesses, deficits, or as an abnormal distribution of fluids within the body.2 Excesses result from increased intake and decreased loss of fluid and electrolytes. Deficits result from abnormal shifts of fluid and electrolytes among the intravascular and extravascular fluid compartments of the body. Excesses can occur with kidney dysfunction, promoting fluid retention, and with respiratory dysfunction, which promotes carbon dioxide retention. Deficits are commonly associated with reduced intake of fluids and nutrition. Diaphoresis and wounds can also contribute to major fluid loss. Diarrhea and vomiting drain the gastrointestinal tract of fluid. Hemorrhage always results in fluid and electrolyte loss. Deficits may be secondary to fluid entrapment and localized edema within the body (third-spacing), making this source of fluid unavailable for regulation of homeostasis. Assessment of fluid and electrolyte balance is based on both subjective and objective findings (Table 16-1). At the bedside, the physical therapist must be alert to complaints of headache, thirst, and nausea, as well as changes in dyspnea, skin turgor, and muscle strength. More objective assessment is based on fluid intake, output, and body weight. Fluid balance is so critical to physical well-being and cardiovascular and pulmonary sufficiency that fluid input and output records are routinely maintained at bedside. These records also include fluid volume lost in urine and feces, wound drainage, and fluids aspirated from any body cavity (e.g., abdomen and pleural space). Table 16-1 Assessment of Fluid and Electrolyte Imbalance Modified from Phipps WJ, Long BC, Woods NF, editors: Medical-surgical nursing: Concepts and clinical practice, ed 6, Philadelphia, 1999, Elsevier. Chest tubes are large catheters placed in the pleural cavity to evacuate fluid and air and to drain into a graduated collection reservoir at bedside.2 A typical chest tube drainage and collection system is shown in Figure 16-4, A. The removal of thick fluids such as blood and organized exudates by chest tubes is often indicated to prevent entrapment and loculation. Chest tubes are commonly inserted in the sixth intercostal space in the mid- or posterior axillary line. Chest tubes inserted into the pleural space are used to evacuate air or exudate. Chest tubes can also be inserted into the mediastinum to evacuate blood such as after open heart surgery (Figure 16-4, B). Control of acid-base balance in the body is achieved by regulation of the hydrogen ion concentration in the body fluids.3,4 The pH of the body is normally maintained within a narrow range of 7.35 to 7.45 or slightly alkaline. When pH of the blood drops below 7.35, a state of acidosis exists; above 7.45, a state of alkalosis exists. Regulation of pH is vital because even slight deviations from the normal range cause marked changes in the rate of cellular chemical reactions. A pH below 6.8 or above 8 is incompatible with life. Acid-base balance is controlled by several regulatory buffer systems, primarily the carbonic acid-bicarbonate, phosphate, and protein buffer systems. These systems act very quickly to prevent moment-to-moment changes in pH. In compensation, pH is returned to normal by altering the component not primarily affected. If the primary cause is respiratory, the compensating mechanism is metabolic. If the primary cause is metabolic, the compensating mechanism is respiratory. The lungs compensate for metabolic problems over hours, whereas the kidneys compensate for respiratory problems over days (see Chapter 10). A guide to the clinical presentation of acid-base imbalances is shown in Table 16-2. Along with the major distinguishing characteristics of acid-base imbalance described in this chapter and elsewhere in this volume, potassium excess (hyperkalemia) is associated with both respiratory and metabolic acidosis, and neuromuscular hyperexcitability is associated with both respiratory and metabolic alkalosis. Table 16-2 Signs and Symptoms of Common Acid-Base Disturbances Analysis of the composition of arterial and mixed venous blood provides vital information about respiratory, cardiac, and metabolic function (see Chapter 10).5,6 For this reason, blood gases are usually analyzed in the ICU. In cases in which the patient’s condition is changing for better or worse over a short period of time or when a specific treatment response is of interest, blood gases may be analyzed several times daily. With an arterial line in place, frequent blood gas analysis is feasible and not traumatic for the patient. Should the patient be anemic, however, blood loss associated with repeated arterial blood sampling may mean that it is contraindicated. Thus requests for arterial blood gas analysis need to be particularly stringent in patients who are anemic. Arterial saturation (SaO2), the proportion of hemoglobin combined with oxygen, can be readily monitored noninvasively by a pulse oximeter (SpO2). The earlobe or a finger is warmed by rubbing before the oximeter sensor is attached. Within seconds, the SpO2 can be read directly from the monitor. Pulse oximetry is a useful adjunct for routine evaluation of the effectiveness of mechanical ventilation, the effect of anesthesia, and the treatment response. Continuous estimation of SaO2 is particularly useful before, during, and after mobilization and exercise, position changes, and other therapeutic interventions. The SaO2 may appear to be reduced in patients who are anemic or jaundiced or those who have reduced cardiac output. The SpO2 reading may be inaccurate in patients with poor peripheral perfusion, who have cold extremities, or who have pigmented skin. The oxygenation of patients in the ICU varies considerably over time and even moment-to-moment, irrespective of sedation, high post end-expiratory pressure, or inverse ventilation.7 Mixed venous oxygen saturation (
Monitoring Systems, Catheters, and Devices in the Intensive Care Unit
Fluid and Electrolyte Balance
Area
Fluid Excess/Electrolyte Imbalance
Fluid Loss/Electrolyte Imbalance
Head and neck
Distended neck veins, facial edema
Thirst, dry mucous membranes
Extremities
Dependent edema “pitting,” discomfort from weight of bed covers
Muscle weakness, tingling, tetany
Skin
Warm, moist, taut, cool feeling when edematous
Dry, decreased turgor
Respiration
Dyspnea, orthopnea, productive cough, moist breath sounds
Changes in rate and depth of breathing
Circulation
Hypertension, jugular pulse visible at 45-degree sitting angle, atrial dysrhythmias
Pulse rate irregularities, dysrhythmia, postural hypotension, sinus tachycardia
Abdomen
Increased girth, fluid wave
Abdominal cramps
Chest Tube Drainage and Fluid Collection Systems
Acid-Base Balance
Respiratory Disturbances
Metabolic Disturbances
Acidosis
Alkalosis
Blood Gases
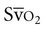 ) provides a useful index of oxygen delivery and utilization at the tissue level.8
) provides a useful index of oxygen delivery and utilization at the tissue level.8![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine