48 Mitral Valvuloplasty
 Introduction
Introduction
Until the first publication by Inoue and coworkers describing percutaneous mitral commissurotomy (PMC) in 1984, surgery was the only treatment for patients with mitral stenosis.1 Since then, the technique has evolved considerably. A large number of patients with a wide range of clinical conditions have now been treated, enabling efficacy and risk to be assessed; long-term results are now available, so it is possible to select the most appropriate candidates for treatment by this method.2 As expected from the earlier experience with closed surgical commissurotomy, the positive immediate as well as long-term results obtained during this period have led to increased worldwide use of the technique. We begin this chapter with a report of our own experience and then review the data available in the literature.
 Personal Experience
Personal Experience
Patients
We have attempted PMC in 2773 patients, whose mean age was 47 ± 15 years (range, 9–86 years).3 Altogether, 71% were in class III or IV, according to the classification system of the New York Heart Association (NYHA), and 31% were in atrial fibrillation (AF). After fluoroscopic examination and echocardiography, the patients were divided into the following anatomic groups for selection of the most adequate treatments:
Results
Immediate Hemodynamic and Echocardiographic Results
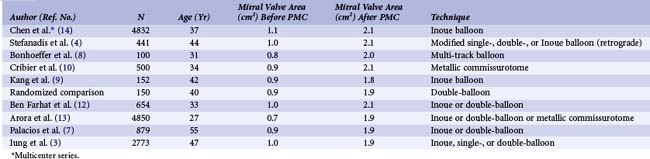
Successful PMC brought immediate hemodynamic improvement, as shown in Table 48-1. Echocardiographic techniques confirmed the results obtained by hemodynamics. The valve area increased from 1.0 ± 0.2 to 1.9 ± 0.3 cm2, as assessed by two-dimensional echocardiography. Poor results, as defined by a valve area less than 1.5 cm2 or mitral regurgitation greater than 2 or 4 (or both), occurred in 11.5% of patients.
 Devices and Techniques
Devices and Techniques
PMC acts in the same way as surgical commissurotomy, by opening the fused commissures (Fig. 48-1). PMC is of little or no help in cases of restricted valvular mobility caused by valve fibrosis or severe subvalvular disease. The techniques and devices used for PMC have varied over time and from group to group. At the present time, there are two approaches—trans-arterial and trans-venous—and two main techniques—the double-balloon technique and the Inoue technique.
Trans-arterial or Retrograde Approach
The trans-arterial, or retrograde, approach has the advantage of minimizing or eliminating the risk of atrial septal defect (ASD) and the disadvantage of potential arterial damage. This technique has now been abandoned because of its complexity. The retrograde technique without trans-septal catheterization has been used with good results and no serious complications, but its use is not widespread.4
Trans-venous or Antegrade Approach
The trans-venous, or antegrade, approach is more widely used. It is performed through the femoral vein or, exceptionally, through the jugular vein. Trans-septal catheterization is the first and one of the most crucial steps of the procedure. The conditions necessary for safe and successful trans-septal catheterization are (1) knowledge of the anatomy, which may be modified in patients with mitral stenosis, in whom normal geometry has been lost because both atria are enlarged and the convexity of the septum is exaggerated; (2) knowledge of contraindications; and (3) experience of the operators acquired by performance of the technique on a regular basis. Usually, trans-septal catheterization is performed under fluoroscopic guidance, ideally with use of several views. Continuous pressure monitoring is recommended. Echocardiography is not systematic during trans-septal catheterization; however, it has the potential to enhance its safety, especially in the early part of the operator’s experience. This has been done primarily with TEE, which is superior to trans-thoracic echocardiography (TTE) for imaging the inter-atrial septum (IAS). Nevertheless, the trans-esophageal approach is not easy in the catheterization laboratory (cath lab) and should probably be restricted to cases in which technical difficulties are encountered (e.g., severe anatomic distortion) (see Video 48-1 ![]() ). Intracardiac echocardiography (ICE) is currently considered the imaging tool of choice in several catheterization centers to guide trans-septal puncture because it may be used without additional operators or general anesthesia, although the price of the device seriously limits its use in most places.5 Recent data suggest that the real-time three-dimensional trans-esophageal technique (RT 3DTEE) may further improve visualization of the septum and assessment of tenting during septal puncture.6
). Intracardiac echocardiography (ICE) is currently considered the imaging tool of choice in several catheterization centers to guide trans-septal puncture because it may be used without additional operators or general anesthesia, although the price of the device seriously limits its use in most places.5 Recent data suggest that the real-time three-dimensional trans-esophageal technique (RT 3DTEE) may further improve visualization of the septum and assessment of tenting during septal puncture.6
Double-Balloon Technique
The double-balloon technique is one of the two main balloon techniques in current use; it has been described extensively.7 Briefly, the technique is as follows: After trans-septal catheterization, the left ventricle is catheterized with the use of a floating balloon catheter. One or two long exchange guidewires are positioned in the apex of the left ventricle or, less frequently, in the ascending aorta. The IAS is dilated with the use of a peripheral angioplasty balloon (8 or 6 mm in diameter). Finally, the balloons (15 to 20 mm in diameter) are positioned across the mitral valve. The multi-track system is a more recent refinement of the double-balloon technique that uses a monorail system, requiring the presence of only one guidewire and easing the performance of the dilation compared with the standard double-balloon technique. Clinical experience with this device is, however, limited.8
Inoue Technique
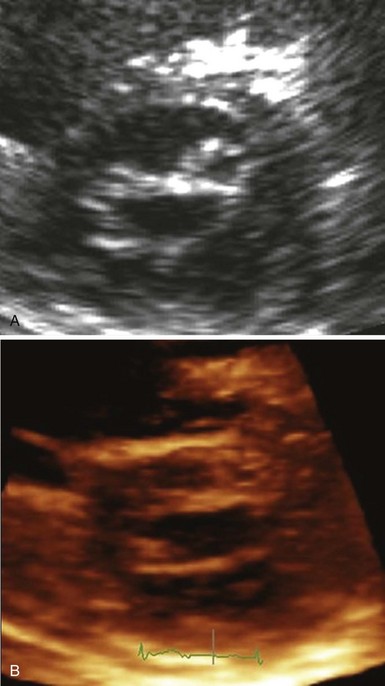
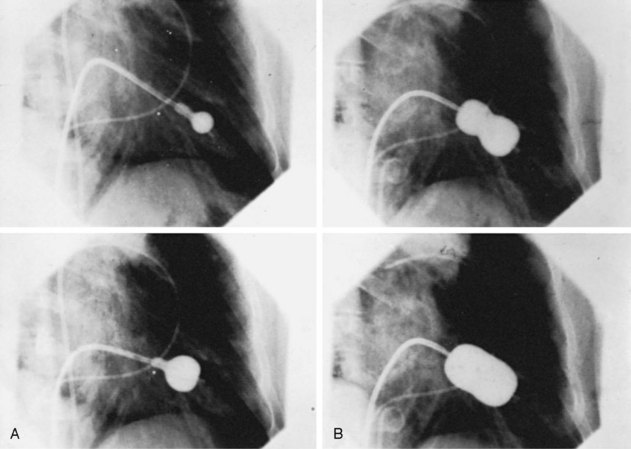
The Inoue technique was the first to be described, and wide experience has now been acquired by a number of groups worldwide.1,3,9 The Inoue balloon, composed of nylon and rubber micromesh, is self-positioning and pressure extensible. It is large (24 to 30 mm in diameter) and has a low profile (4.5 mm). The balloon has three distinct parts, each with a specific elasticity, enabling them to be inflated sequentially. This sequence allows fast, stable positioning across the valve. There are four sizes of the Inoue balloon (24 mm, 26 mm, 28 mm, and 30 mm); each is pressure dependent, so its diameter can be varied by up to 4 mm, as required by circumstances. The main steps are as follows: After trans-septal catheterization, a stiff guidewire is introduced into the left atrium. The femoral entry site and the atrial septum are dilated with a rigid dilator (14 French [F]), and the balloon is introduced into the left atrium. Inoue recommended the use of a stepwise dilation technique under echocardiographic guidance. Balloon size is chosen in accordance with the patient’s height (26 mm in very small patients or infants, 28 mm in patients shorter than 1.60 m, and 30 mm in patients taller than 1.60 m). The balloon is inflated sequentially. First, the distal portion is inflated with 1 or 2 mL of a diluted contrast medium; it acts as a floating balloon catheter when crossing the mitral valve. Second, the distal part is further inflated, and the balloon is pulled back into the mitral orifice. Inflation then occurs at the level of the proximal part and finally in the central portion, with the disappearance of the central waist at full inflation (Fig. 48-2; see Videos 48-2 and 48-3 ![]() ).
).
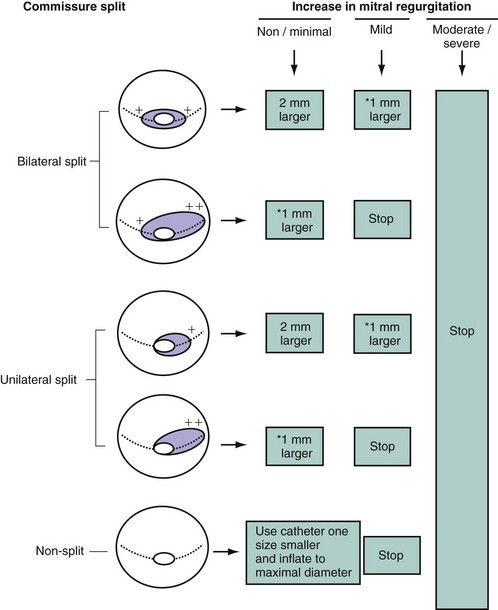
The first inflation is performed 4 mm below the maximal balloon size, and the balloon size is increased in steps of 1 mm each. The balloon is then deflated and withdrawn into the left atrium. If mitral regurgitation (assessed by color Doppler echocardiography) has not increased by more than ¼ and the valve area is less than 1 cm2/m2 of body surface area, the balloon is re-advanced across the valve, and PMC is repeated with a balloon diameter increased by 1 mm (Fig. 48-3).5 The criteria for ending are an adequate valve area or an increase in the degree of mitral regurgitation. Data currently available comparing the double-balloon and Inoue techniques suggest that the Inoue technique eases the procedure and has equivalent efficacy and lower risk.9 In fact, the Inoue technique has already become the most popular in the world, having been used in more than 10,000 patients. Finally, even though randomized studies are lacking and intra-procedural echocardiography lacks practicality, the stepwise technique under echocardiographic guidance certainly allows the best use of the mechanical properties of the Inoue balloon and therefore optimizes the results.
Metallic Commissurotome
During the late 1990s, Cribier and colleagues introduced the metallic commissurotome, which uses a device similar to the Tubb dilator used during closed commissurotomy.10 Experience with this device comprises about 1000 cases, mainly from developing countries. The initial results suggest that the technique has efficacy similar to that of balloon commissurotomy, but the risk of hemopericardium seems higher owing to the presence of a stiff guidewire in the left ventricle. In addition, this technique is more demanding on the operator compared with the Inoue technique. The potential advantage is that the dilator is reusable, which reduces the cost of the procedure. Its use is currently very limited, if any.
 Monitoring of the Procedure and Evaluation of Immediate Results
Monitoring of the Procedure and Evaluation of Immediate Results
Monitoring of the Procedure
Trans-thoracic echocardiography (TTE) is the preferred technique for echocardiographic monitoring in most centers. Trans-esophageal guidance under general anesthesia, although performed systematically in some centers, is restricted in others to cases in which difficulty is encountered or in pregnant patients to reduce radiation exposure. TEE provides excellent views of the position of the balloon as it is advanced into the mitral orifice. TEE may also better assess the severity and mechanism of mitral regurgitation and residual shunting. The recently introduced real-time three-dimensional technique has the added advantage of providing an “en face” view of the mitral valve, allowing better visualization of the trajectory and positioning of the balloon before inflation.6 ICE is another alternative that helps monitor balloon positioning and inflation. Although the visualization of the mitral orifice is less optimal with ICE than with TEE, adequate views may be obtained from the right ventricle.5 The following guidelines have been suggested for monitoring the procedure: First, use of the mean left atrial pressure and mean valve gradient can be criticized because of variations that may occur, particularly with respect to changes in heart rate or cardiac output. Second, repeated evaluation of the valve area during the procedure by hemodynamic measurements lacks practicality and may be subject to error because of the instability of the patient’s condition and the inaccuracy of Gorlin’s formula in the presence of atrial shunts or mitral regurgitation. The accuracy of Doppler measurements during valvuloplasty is low, so planimetry from two-dimensional echocardiography appears to be the method of choice if it is technically feasible. Color Doppler assessment is the method of choice for sequential evaluation of the changes in the degree of regurgitation. The commissural opening, which is the main parameter, is usually assessed in the parasternal short-axis view during TTE. Real-time three-dimensional echocardiography is the most accurate method for assessing the degree of opening using short-axis views or real-time three-dimensional TEE en face views, which may provide further information regarding the extent of the commissural opening (Video 48-4 ![]() ).6,11 The following criteria have been proposed for the desired endpoint of the procedure: (1) mitral valve area of more than 1 cm2/m2 of the body surface area; (2) complete opening of at least one commissure; or (3) appearance or increment of regurgitation greater than ¼. It is vital that the strategy be tailored to individual circumstances, taking into account clinical as well as anatomic factors and the cumulative data of peri-procedural monitoring. For example, balloon size, increments of size, and expected final valve area are smaller in certain clinical subsets such as older or pregnant patients, in whom the need for emergency surgery is of concern, and in the presence of tight mitral stenosis, extensive valve or subvalvular disease, and nodular commissural calcification. After the procedure, the most accurate evaluation of the valve area is achieved by echocardiography. To allow for the slight loss during the first 24 hours, this should be performed 1 to 2 days after mitral valvuloplasty, when the valve area may be calculated by planimetry or by the half-pressure time or continuity equation method. The degree of regurgitation may be finally assessed by angiography or color Doppler flow. The most sensitive method for assessing shunting is color Doppler flow, especially when TEE is used; In current practice, the use of postvalvuloplasty TEE is restricted to patients with severe mitral regurgitation to evaluate the mechanisms. In experienced centers, the procedure can be performed using a single venous approach and noninvasive monitoring, which reduces the risk, discomfort, and costs.
).6,11 The following criteria have been proposed for the desired endpoint of the procedure: (1) mitral valve area of more than 1 cm2/m2 of the body surface area; (2) complete opening of at least one commissure; or (3) appearance or increment of regurgitation greater than ¼. It is vital that the strategy be tailored to individual circumstances, taking into account clinical as well as anatomic factors and the cumulative data of peri-procedural monitoring. For example, balloon size, increments of size, and expected final valve area are smaller in certain clinical subsets such as older or pregnant patients, in whom the need for emergency surgery is of concern, and in the presence of tight mitral stenosis, extensive valve or subvalvular disease, and nodular commissural calcification. After the procedure, the most accurate evaluation of the valve area is achieved by echocardiography. To allow for the slight loss during the first 24 hours, this should be performed 1 to 2 days after mitral valvuloplasty, when the valve area may be calculated by planimetry or by the half-pressure time or continuity equation method. The degree of regurgitation may be finally assessed by angiography or color Doppler flow. The most sensitive method for assessing shunting is color Doppler flow, especially when TEE is used; In current practice, the use of postvalvuloplasty TEE is restricted to patients with severe mitral regurgitation to evaluate the mechanisms. In experienced centers, the procedure can be performed using a single venous approach and noninvasive monitoring, which reduces the risk, discomfort, and costs.
Immediate Results
Failures
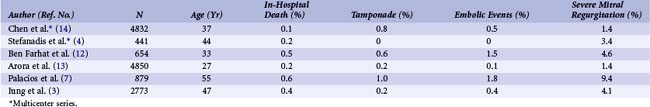
The failure rate ranges from 1% to 17%.3,4,7–10,12–14 Failure is often caused by an inability to puncture the atrial septum or position the balloon correctly across the valve. Most failures occur early in the operator’s experience. Failures can also result from unfavorable anatomy such as severe atrial stenosis or predominant subvalvular stenosis.
Hemodynamics
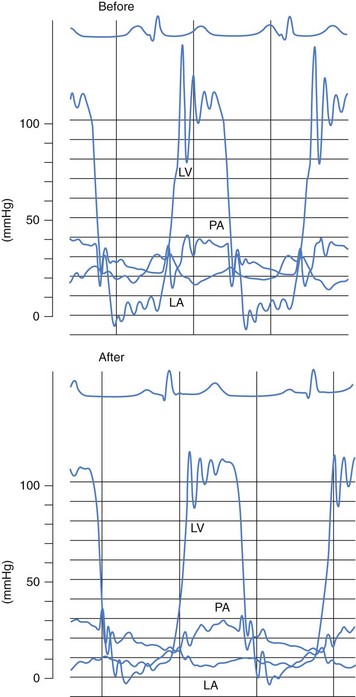
Our results, like those of others, demonstrate the efficacy of PMC, which usually provides an increase of more than 100% in the valve area (Table 48-2). The improvement in valve function results in an immediate decrease in left atrial pressure (Fig. 48-4) and a slight increase in cardiac index. A gradual decrease in pulmonary arterial pressure and pulmonary vascular resistance is seen. High pulmonary vascular resistance continues to decrease in the absence of re-stenosis. PMC has a beneficial effect on exercise capacity. In addition, studies have shown that this technique improves the pump function of the left atrium and the LAA and decreases left atrial stiffness. It also results in a decrease in the intensity of spontaneous echocardiographic contrast in the left atrium.
Complications
Large series have enabled assessment of the risks in the technique (Table 48-3).3,4,7–10,12–14 Procedural mortality has ranged from 0% to 3% in most series. The main causes of death are left ventricular perforation and poor general condition of the patient. The incidence of hemopericardium has varied from 0.5% to 12%. Pericardial hemorrhage may be related to trans-septal catheterization or to apex perforation by guidewires or the balloon itself when exaggerated movement occurs with the over-the-wire techniques; however, this complication is virtually eliminated with the Inoue balloon technique. If hypotension occurs during PMC, hemopericardium must be suspected and echocardiography immediately performed. Pericardiocentesis in the cath lab, ideally under echocardiographic guidance, usually allows stabilization of the patient’s condition and secondary transfer for cardiac surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree