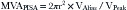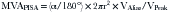5 Mitral Stenosis
Goals of Echocardiography in Mitral Stenosis
 To establish that mitral stenosis (MS) is present
To establish that mitral stenosis (MS) is present
 To establish the level of obstruction
To establish the level of obstruction
 To establish the hemodynamic parameters: gradient, area, cardiac index (CI), right ventricular systolic pressure (RVSP)
To establish the hemodynamic parameters: gradient, area, cardiac index (CI), right ventricular systolic pressure (RVSP)
 To establish suitability for valvuloplasty
To establish suitability for valvuloplasty
 To identify complications of mitral stenosis
To identify complications of mitral stenosis
 To identify concurrent valvulopathies/pathologies
To identify concurrent valvulopathies/pathologies
Scanning Issues
Required Parameters to Obtain from Scanning
 Right ventricle size and systolic function
Right ventricle size and systolic function
 Indirect pressure overload signs: left atrium (LA) size, RVSP
Indirect pressure overload signs: left atrium (LA) size, RVSP
 Left ventricular outflow tract (LVOT)–derived cardiac output and stroke volume. Note that in the presence of AI, the stroke volume measured in the LVOT is the total forward stroke volume, not the net forward stroke volume.
Left ventricular outflow tract (LVOT)–derived cardiac output and stroke volume. Note that in the presence of AI, the stroke volume measured in the LVOT is the total forward stroke volume, not the net forward stroke volume.
 Height and weight for body surface area
Height and weight for body surface area
Valid Methods for Mitral Valve Area Determination (Need ≥2 Concordant)
Scanning Notes
Gradient
 Ensure that transmitral Doppler sampling is correctly aligned, and that continuous wave Doppler is being used with adequate gain settings.
Ensure that transmitral Doppler sampling is correctly aligned, and that continuous wave Doppler is being used with adequate gain settings.
 If sinus rhythm is present, measure three spectral profiles.
If sinus rhythm is present, measure three spectral profiles.
 If atrial fibrillation is detected, measure five spectral profiles.
If atrial fibrillation is detected, measure five spectral profiles.
 Ideally, measure 10 spectral profiles.
Ideally, measure 10 spectral profiles.
 Spectral profiles should be displayed at two-thirds the height of the display, and wide enough so that there are two or three per display.
Spectral profiles should be displayed at two-thirds the height of the display, and wide enough so that there are two or three per display.
 If the gradient is not severe, but the area is, consider provocative maneuvers (e.g., sit-ups) to increase flow and (thereby the gradient).
If the gradient is not severe, but the area is, consider provocative maneuvers (e.g., sit-ups) to increase flow and (thereby the gradient).
Planimetry
 Optimize the gain settings for planimetry—over-gain results in a falsely low estimation of mitral valve area (MVA) due to “blooming” of the margins.
Optimize the gain settings for planimetry—over-gain results in a falsely low estimation of mitral valve area (MVA) due to “blooming” of the margins.
 Measure the area at the narrowest part of the funnel-like orifice of MS—this may be at the level of the leaflet tips or at the subvalvar level.
Measure the area at the narrowest part of the funnel-like orifice of MS—this may be at the level of the leaflet tips or at the subvalvar level.
 Be cautious if there was a prior commissurotomy—planimetry underrepresents area if the orifice has deep “splits” into the commissures (or leaflets), which are difficult so see by ultrasound, especially if they extend off the plane of imaging.
Be cautious if there was a prior commissurotomy—planimetry underrepresents area if the orifice has deep “splits” into the commissures (or leaflets), which are difficult so see by ultrasound, especially if they extend off the plane of imaging.
Pressure Half Time
All are factors that reduce the accuracy of the PHT relation to MVA.
Proximal Isovelocity Surface Area
where r = PISA radius.
The PISA method is based on the assumption that there is a hemisphere of flow acceleration such as would occur with a planar orifice. This often is not the case in mitral stenosis, however, particularly when the greatest stenosis is subvalvar. To adjust for the effect of the orifice on the volume of the PISA, use of an angle correction factor has been suggested (Fig. 5-1), multiplying the preceding calculation by α/180°. Not all echo systems carry angle measurement software.
where α = the measured angle of the “plane” of the orifice and r = PISA radius.
Reporting Issues
Gradient Issues
 If there is a difference in gradient between current and previous echocardiographic determinations, then review the following variables:
If there is a difference in gradient between current and previous echocardiographic determinations, then review the following variables:
 Recall that the accepted gradients for “severe” assume the absence of factors provoking higher output, such as the following:
Recall that the accepted gradients for “severe” assume the absence of factors provoking higher output, such as the following:
 The 95% CI for mitral valve gradient is 3 mm Hg (vs. wedge:LV recordings).
The 95% CI for mitral valve gradient is 3 mm Hg (vs. wedge:LV recordings).
 Direct left atrial pressure recording (LA:LV) typically leads to a lower mitral gradient, as it is without the phase delay of pulmonary capillary wedge tracings.
Direct left atrial pressure recording (LA:LV) typically leads to a lower mitral gradient, as it is without the phase delay of pulmonary capillary wedge tracings.
Area Issues
 Place less emphasis on the valve area, which is invariably calculated (other than by the planimetry method) and, therefore, is subject to larger error.
Place less emphasis on the valve area, which is invariably calculated (other than by the planimetry method) and, therefore, is subject to larger error.
Pressure Half Time Method
 The PHT relation (220) was validated in a relatively small series and is best applied for MVA between 1 and 1.5 cm2; r = 0.75.1 The actual precision of the correlation is less than has been thought.
The PHT relation (220) was validated in a relatively small series and is best applied for MVA between 1 and 1.5 cm2; r = 0.75.1 The actual precision of the correlation is less than has been thought.
 The 220 constant is actually different for both MVA <1 cm2 and MVA >1.5 cm2; therefore, use of the relationship at higher or lower areas is essentially an extrapolation of the known relationship, and assumes that a known variable (220) is a constant, which it is not.
The 220 constant is actually different for both MVA <1 cm2 and MVA >1.5 cm2; therefore, use of the relationship at higher or lower areas is essentially an extrapolation of the known relationship, and assumes that a known variable (220) is a constant, which it is not.
 In the presence of AI, there are two ventricular inflows determining the spectral inflow:diastolic relationship. The PHT relation, therefore, is less representative of MS alone, in the presence of more than moderate AI. Correlation and SEE for MVA by PHT (vs. Gorlin) for patients both without and with AI are as follows:
In the presence of AI, there are two ventricular inflows determining the spectral inflow:diastolic relationship. The PHT relation, therefore, is less representative of MS alone, in the presence of more than moderate AI. Correlation and SEE for MVA by PHT (vs. Gorlin) for patients both without and with AI are as follows:
 PHT is less accurate in patients older than 65 years of age, because of effects of hypertension on the ventricular diastolic properties, leading to overestimation of MVA.3
PHT is less accurate in patients older than 65 years of age, because of effects of hypertension on the ventricular diastolic properties, leading to overestimation of MVA.3
 PHT is less accurate for 3 months after CBV,4 because passive ventricular diastolic properties and atrial diastolic properties are adapting to the changes in load (i.e., less atrial/more ventricular).
PHT is less accurate for 3 months after CBV,4 because passive ventricular diastolic properties and atrial diastolic properties are adapting to the changes in load (i.e., less atrial/more ventricular).
Planimetry Method
 When images are amenable to planimetry, it is an accurate technique. It requires attention to scan extensively along the short axis of the orifice, to ensure sampling at the narrowest part. This may be at the leaflet tips, or in the subvalvar zone, which is important not to miss.
When images are amenable to planimetry, it is an accurate technique. It requires attention to scan extensively along the short axis of the orifice, to ensure sampling at the narrowest part. This may be at the leaflet tips, or in the subvalvar zone, which is important not to miss.
 Correlation for MVA by planimetry (vs. cardiac catheterization): r = 0.92 – 0.95.5,6
Correlation for MVA by planimetry (vs. cardiac catheterization): r = 0.92 – 0.95.5,6
 Planimetry of MVA is an AI-independent technique.
Planimetry of MVA is an AI-independent technique.
 Planimetry is less accurate with prior commissurotomy,3,7,8 because the split often is eccentric: it may lead out onto another plane of imaging and be missed, or may go into a bright commissure that is difficult to visualize.
Planimetry is less accurate with prior commissurotomy,3,7,8 because the split often is eccentric: it may lead out onto another plane of imaging and be missed, or may go into a bright commissure that is difficult to visualize.
Continuity Method
 MVA by the continuity method is less accurate when the ideal reference site (the LVOT) has more than mild AI. The tricuspid reference site is harder to use, and is at least as likely to have TR. For all-comers, the correlation of continuity is very good: r = 0.91, SEE = 0.24 cm2,2 especially in the absence of AI: r = 0.93.2 However, in the presence of AI it is less: r = 0.84.2
MVA by the continuity method is less accurate when the ideal reference site (the LVOT) has more than mild AI. The tricuspid reference site is harder to use, and is at least as likely to have TR. For all-comers, the correlation of continuity is very good: r = 0.91, SEE = 0.24 cm2,2 especially in the absence of AI: r = 0.93.2 However, in the presence of AI it is less: r = 0.84.2
Proximal Isovelocity Surface Area Method
Real-Time Three-Dimensional Echocardiography
The real-time 3D technique allows depiction of the mitral orifice on any plane, and thus should assist with the task of planimetry. Although studies are limited to date, real-time 3D echocardiography shows very good correlation with Gorlin (average difference of 0.08 cm2), and low inter- and intraobserver variability (κ of 0.84 and 0.96, respectively), which in comparative studies was better than with other methods.10
Descriptors of Mitral Stenosis Severity
 After it has been established that MS is present, the next important task is to determine its severity. This involves a composite assessment of symptoms, physical findings, and hemodynamics. It must be recalled that MVA < 1 cm2 always indicates severe MS, but larger patients or those with higher cardiac output (CO) needs are commonly “severe” above an area of 1 cm2 and receive intervention for average MVAs of 1.2 or 1.3 cm2.
After it has been established that MS is present, the next important task is to determine its severity. This involves a composite assessment of symptoms, physical findings, and hemodynamics. It must be recalled that MVA < 1 cm2 always indicates severe MS, but larger patients or those with higher cardiac output (CO) needs are commonly “severe” above an area of 1 cm2 and receive intervention for average MVAs of 1.2 or 1.3 cm2.
 Distinguishing moderate from mild is really of little consequence, as both are “nonsevere” and would not prompt intervention.
Distinguishing moderate from mild is really of little consequence, as both are “nonsevere” and would not prompt intervention.
 “Severe” = mean gradient >12 mm Hg at rest, and an area of <1.3 cm2.
“Severe” = mean gradient >12 mm Hg at rest, and an area of <1.3 cm2.
 “Critical” = mean gradient > 12 mm Hg at rest, and an area of <1.0 cm2
“Critical” = mean gradient > 12 mm Hg at rest, and an area of <1.0 cm2
 Moderate = mean gradient < 12 mm Hg at rest, and an area of ≥1.3 cm2
Moderate = mean gradient < 12 mm Hg at rest, and an area of ≥1.3 cm2
Suitability for Valvuloplasty and Commissurotomy Issues
 Suitability for commissurotomy is determined by the following:
Suitability for commissurotomy is determined by the following:
 Calcification, especially of commissures, is a marker of higher short- and long-term mortality with CBV.
Calcification, especially of commissures, is a marker of higher short- and long-term mortality with CBV.
 Commissurotomy is generally unsuitable if there is MR >2+.
Commissurotomy is generally unsuitable if there is MR >2+.
 There is substantial risk of arterial embolism if there is clot within the body of the left atrium, because valvuloplasty wires invariably are in the body of the atrium. There is a smaller risk of embolism if clot is within the LAA, because the wires that must traverse the LA body may avoid the LAA. TEE is clearly superior to evaluate for the presence of LA and LAA clot.
There is substantial risk of arterial embolism if there is clot within the body of the left atrium, because valvuloplasty wires invariably are in the body of the atrium. There is a smaller risk of embolism if clot is within the LAA, because the wires that must traverse the LA body may avoid the LAA. TEE is clearly superior to evaluate for the presence of LA and LAA clot.
Surgical Issues
 Establish the degree of submitral (annular) calcification. There is a greater risk of periprosthesis (paravalvar) MR when submitral (annular) calcification is severe.
Establish the degree of submitral (annular) calcification. There is a greater risk of periprosthesis (paravalvar) MR when submitral (annular) calcification is severe.
 Determine the degree of AI: in severe MR, IABP should not be used if there is concurrent AI ≥2+.
Determine the degree of AI: in severe MR, IABP should not be used if there is concurrent AI ≥2+.
 Describe the RV in detail (RVH/systolic dysfunction/size), as well as the RVSP.
Describe the RV in detail (RVH/systolic dysfunction/size), as well as the RVSP.
 RA size is a reasonable descriptor of RV diastolic function, if neither TR nor atrial fibrillation is present.
RA size is a reasonable descriptor of RV diastolic function, if neither TR nor atrial fibrillation is present.
Provocative Testing to Enhance Transmitral Gradients and Right Ventricular Systolic Pressure
Simple bedside maneuvers (e.g., a few sit-ups) or more sophisticated ones (e.g., supine bicycle, upright treadmill testing, or dobutamine) may be used to increase the heart rate and cardiac output, and thereby enhance the mitral gradient and RVSP. The role of such testing is unclear: in mitral stenosis the gradient would be expected to increase, and generally it does, about two-fold, when patients are subjected to a Bruce or modified Bruce protocol: from 9 ± 7 mm Hg at rest to 17 ± 8 mm Hg, as does the RVSP, from 41 ±1 9 mm Hg to 70 ± 32 mm Hg.11 The optimal interpretation of the provoked hemodynamics is unclear. Advocates suggest that it better describes the basis of exertional symptoms and may be contributory when the resting hemodynamics are less striking than the (exertional) symptoms and findings.
Dobutamine stress echo (a gradient cut-off of 18 mm Hg) has been shown to predict clinical events (90% sensitivity, 87% specificity, and 90% accuracy) over a 5-year period, and increases the detection of medium- and higher-risk cases.12 Dobutamine stress echocardiography may assist with the evaluation of patients whose symptoms are out of proportion to the calculated valve area, and who may benefit from “medical or invasive intervention.”13
Notes on Mitral Stenosis
Etiologies of Mitral Stenosis
Acquired
 Rheumatic origin is by far the most common cause of MS (>99% of all cases).
Rheumatic origin is by far the most common cause of MS (>99% of all cases).
 Severe mitral annular calcification may cause mild/moderate MS but very rarely causes severe MS.
Severe mitral annular calcification may cause mild/moderate MS but very rarely causes severe MS.
 LA myxoma/thrombus is an uncommon cause of MS (<1%).
LA myxoma/thrombus is an uncommon cause of MS (<1%).
 A mitral annuloplasty ring may overly narrow the orifice.
A mitral annuloplasty ring may overly narrow the orifice.
 Malignant carcinoid, rheumatoid arthritis, systemic lupus erythematosus, and other tumors are very rare causes.
Malignant carcinoid, rheumatoid arthritis, systemic lupus erythematosus, and other tumors are very rare causes.
Pathophysiology
 The normal mitral valve area is 4.5 to 6 cm2. A diastolic transmitral valve gradient occurs with loss of about half of the original area. There is increased left atrial pressure at rest with MVA of 1.5 cm2.
The normal mitral valve area is 4.5 to 6 cm2. A diastolic transmitral valve gradient occurs with loss of about half of the original area. There is increased left atrial pressure at rest with MVA of 1.5 cm2.
 Two thirds (at least) of MS cases occur in women. Of all cases of rheumatic heart disease, 25% are pure MS and 40% are mixed MS/mitral insufficiency.
Two thirds (at least) of MS cases occur in women. Of all cases of rheumatic heart disease, 25% are pure MS and 40% are mixed MS/mitral insufficiency.
 The course of disease proceeds as follows:
The course of disease proceeds as follows:
Role of Transthoracic and Transesophageal Electrocardiography in Mitral Stenosis
Transthoracic Echocardiography
TEE is better than TTE for the following:
 Detection of LAA and LA thrombus, which is necessary information when contemplating CBV
Detection of LAA and LA thrombus, which is necessary information when contemplating CBV
 Estimation of MR severity (TTE may underestimate MR severity)
Estimation of MR severity (TTE may underestimate MR severity)
TEE is not superior for the evaluation of subvalvar disease. The thickened and often calcified leaflets impair subvalvar imaging from the esophagus. Transgastric views are helpful to circumvent this imaging problem.
Cardiac Catheterization for Assessment of Mitral Stenosis
Usual Technique
Gorlin Equation
 The Gorlin equation is employed, using the following variables: CO, DFP, HR, gradient. These variables are readily obtained at cardiac catheterization.
The Gorlin equation is employed, using the following variables: CO, DFP, HR, gradient. These variables are readily obtained at cardiac catheterization.
 Historically, the Gorlin equation constant was developed for MS, because there is less flow dependence with MS than for AS.14
Historically, the Gorlin equation constant was developed for MS, because there is less flow dependence with MS than for AS.14
 In a small initial validation (11 cases), variation of serial measurements averaged ±0.0 cm2, but ranged from +0.2 to –0.4 cm2.15
In a small initial validation (11 cases), variation of serial measurements averaged ±0.0 cm2, but ranged from +0.2 to –0.4 cm2.15
 In the initial series, autopsy standard was ±0.5 to ±0.1 cm2 and the variation against autopsy was ≤0.2 cm2.14
In the initial series, autopsy standard was ±0.5 to ±0.1 cm2 and the variation against autopsy was ≤0.2 cm2.14
 The Gorlin equation purportedly estimates anatomic, not effective, orifice, because it was validated against surgery and autopsy, but there is evidence that it does not truly reflect anatomic area.
The Gorlin equation purportedly estimates anatomic, not effective, orifice, because it was validated against surgery and autopsy, but there is evidence that it does not truly reflect anatomic area.
 The Gorlin equation is less accurate in the presence of the following:
The Gorlin equation is less accurate in the presence of the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree