Miscellaneous Pathologic Findings in Lung Allografts
Anja C. Roden, M.D.
Allen P. Burke, M.D.
Joseph J. Maleszewski, M.D.
Posttransplant Acute Lung Injury (Primary Graft Dysfunction)
Primary graft dysfunction (PGD) is a syndrome that is presumed secondary to ischemia and reperfusion injury. It is characterized by acute lung injury and occurs within the first 72 hours after lung transplantation. PGD is the most common cause of death within 30 days of transplant. There may be a relation between the duration of nonperfusion of the graft (cold ischemic storage) and the respiratory and circulatory conditions of the donor prior to transplant. The clinical findings are similar to hyperacute rejection in patients with preformed antibodies, which are generally a contraindication for transplant, but may be occult. In studies published after the International Society of Heart and Lung Transplantation (ISHLT) Working Group on Primary Graft Dysfunction published a standardized definition and grading system, the reported incidence of severe PGD ranged between 22% and 32% of transplant patients.1 The mortality and morbidity are high.2 Furthermore, PGD has been associated with decreased functional status and bronchiolitis obliterans syndrome.3
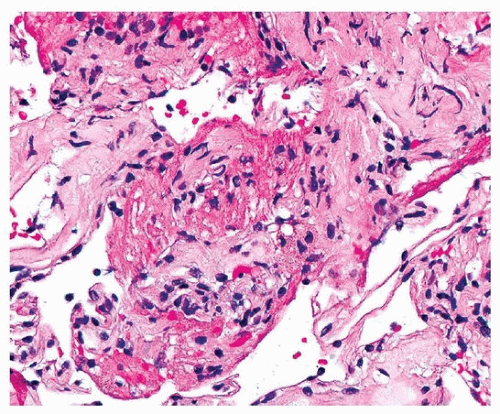
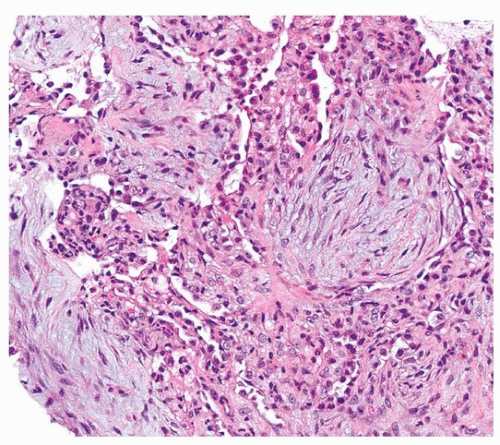
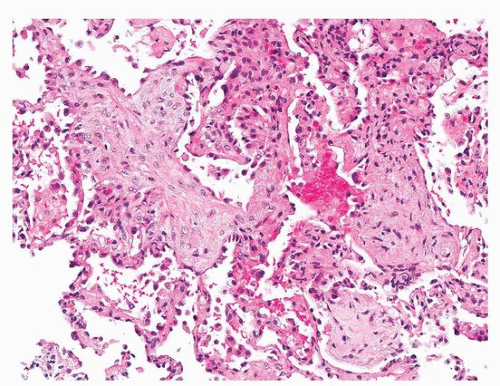
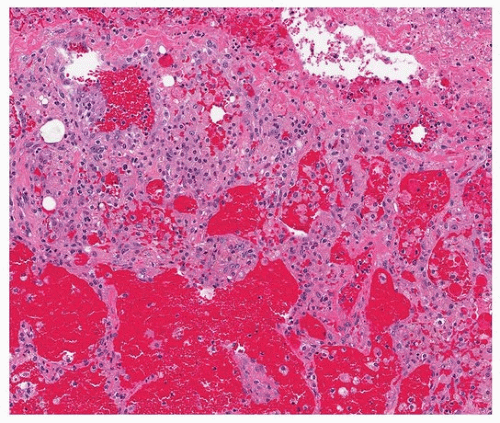
Histologically, PGD manifests as diffuse alveolar damage. Generally, at the time of biopsy, the phase of injury will be organizing, with minimal residual fibrin, thickened interstitium, and reactive endothelial cells (Figs. 54.1, 54.2, 54.3). The differential diagnosis includes acute cellular rejection, although interstitial T-cell infiltration is not prominent in cases of organizing alveolar injury, and there are no perivascular aggregates of lymphocytes. If there are areas of severe ischemia with early infarct, there may be hemorrhage and acute interstitial inflammation, which is difficult to distinguish from hyperacute rejection (Fig. 54.4). The organizing features of diffuse alveolar damage, due to any cause but including PGD, are somewhat similar to organizing pneumonia in that there are nests of proliferating fibroblasts. In general, in organizing diffuse alveolar damage, proliferating fibroblasts are within alveolar septa leading to their expansion, while organizing pneumonia is characterized by intraalveolar and intrabronchiolar polypoid plugs of proliferating fibroblasts (Masson bodies). However, it may be impossible to distinguish organizing pneumonia from organizing diffuse alveolar lung injury on transbronchial biopsy, although the presence of some normal alveoli, without inflammation, edema, or reactive pneumocytes, favors organizing pneumonia.
Lung Infections
Infections are common in lung transplant patients and require consideration in the differential of a patient with suspected rejection (Table 54.1). After graft dysfunction, infections are the second most common cause of death in the first month and the most common cause of death between 31 days and 1 year after transplantation in adults.4 The clinical diagnosis of infection is based largely on culture results. Pathogenicity, titer or colony number, and multiplicity of organisms are important considerations in determining if these organisms are contaminants.
Biopsies are performed in order to exclude acute rejection as a cause for the patient’s graft dysfunction. Bacterial infections generally cause acute exudates, with fibrin and acute and chronic inflammatory cells in the alveolar spaces, a pattern that does not resemble cellular- or antibody-mediated rejection. Viral and fungal infections, however, can mimic acute cellular rejection, with interstitial and perivascular infiltrates of T lymphocytes.5 For this reason, it is impossible to exclude a viral etiology in all histologically diagnosed rejection. Rejection and infection might coexist, which may be suggested in cases of bacterial infections, in which there is both an acute exudate and interstitial T-cell infiltrates suggestive of cellular rejection.
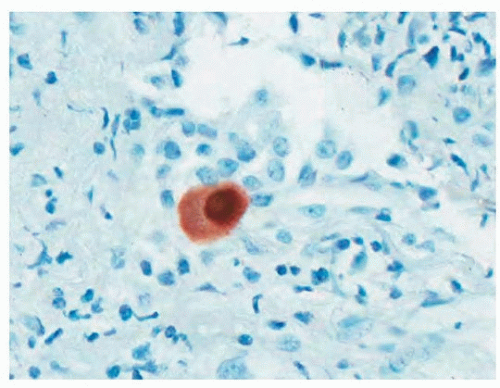
Because cytomegalovirus and pneumocystis are relatively common infections in transplant patients and may be seen readily in biopsy material, it is especially important to exclude these organisms on a routine basis. Immunohistochemical stains for cytomegalovirus (Fig. 54.5) and Gomori methenamine-silver staining for pneumocystis are common diagnostic aids in biopsy samples that complement cytologic preparations of bronchoalveolar lavage fluid. Viral titer quantitation by polymerase chain reaction is of limited benefit in establishing symptomatic infection by cytomegalovirus.6,7
TABLE 54.1 Findings Other Than Rejection to Consider in Lung Allograft | ||
|---|---|---|
|
Mesothelium in Transbronchial Biopsies
Although the acquisition of pleural tissue is rare in transbronchial biopsies,8 it may be somewhat more common in biopsies of allograft lungs, because of the restriction and decreased lung volumes that often occur later in the course of transplant. When reactive, mesothelial cells can mimic carcinoma and necessitate immunohistochemical studies to confirm their origin (Fig. 54.6).
Recurrent Diseases
Of those diseases that commonly lead to transplant, only sarcoidosis often recurs in the allograft lung, without a detriment in survival.9 Histologic features of recurrent sarcoidosis are identical to native lung disease (Fig. 54.7), and the granulomas appear to be derived from recipient cells.10,11 Lymphangioleiomyomatosis recurring in donor lungs has also been reported,12 and, similar to sarcoid, the lesional cells have a recipient origin.13 Other diseases that have been reported to recur in the lung allograft include pulmonary Langerhans cell histiocytosis, giant cell interstitial pneumonia, allergic bronchopulmonary aspergillosis, desquamative interstitial pneumonia, squamous cell and adenocarcinomas, alveolar proteinosis, and panbronchiolitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree