Minimally Invasive Techniques for Coronary Artery Bypass
Emmanuel Moss
Michael E. Halkos
Minimally invasive coronary bypass surgery (MICS) has progressed significantly over the past decade. Patients’ desire to avoid a sternotomy but maintain the survival advantage and durability of LIMA–LAD grafting have fueled these approaches, which have been enabled by significant technologic advances. The earliest form of minimally invasive coronary artery bypass grafting (CABG) was minimally invasive direct coronary artery bypass (MIDCAB), which utilized a small left thoracotomy to harvest the left internal mammary artery (LIMA) under direct vision followed by a hand-sewn anastomosis through the same incision. This technique evolved to include multivessel grafting approaches via left thoracotomy (MICS CABG) for patients with multivessel coronary artery disease (CAD). Thoracoscopic equipment and expertise made way for endoscopic atraumatic CABG (EndoACAB), with endoscopic LIMA harvest followed by a hand-sewn anastomosis through a minithoracotomy. When robotic technology became available, shafted thoracoscopic instruments were replaced by articulating robotic arms to facilitate LIMA harvest (robot-assisted CABG), with most surgeons continuing to perform a manual anastomosis via a minithoracotomy. Some surgeons have mastered a totally endoscopic approach (totally endoscopic coronary artery bypass grafting [TECAB]), in which all portions of the procedure are performed with robotic assistance.
Indications
Indications for minimally invasive surgical revascularization are similar to those of traditional sternotomy CABG. While percutaneous coronary intervention (PCI) is routinely utilized to treat proximal LAD disease, approximately one-half of our patients referred for minimally invasive CABG have single vessel LAD disease. This commonly includes patients with ostial LAD disease, heavy calcification, or complex bifurcation lesions. The remainder have multivessel disease with non-LAD lesions that can be
treated successfully with PCI using a hybrid coronary revascularization (HCR) approach. For robotic-assisted CABG, patients generally fall into two categories: The first is healthy patients wishing to avoid sternotomy but seeking the durability of CABG, while the other involves patients considered high risk for traditional sternotomy CABG but have LAD disease not amenable to PCI or medical therapy.
treated successfully with PCI using a hybrid coronary revascularization (HCR) approach. For robotic-assisted CABG, patients generally fall into two categories: The first is healthy patients wishing to avoid sternotomy but seeking the durability of CABG, while the other involves patients considered high risk for traditional sternotomy CABG but have LAD disease not amenable to PCI or medical therapy.
TABLE 27.1 Favorable and Unfavorable Characteristics to Evaluate When Considering Robotic-Assisted CABG and HCR | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Careful evaluation of the angiogram in collaboration with the cardiologist should focus on the following: (1) Quality and size of the distal LAD; (2) epicardial or intramyocardial LAD course; (3) presence of calcification in the target location; (4) presence of large or nearby diagonal vessels in proximity to the LAD; and (5) additional noncoronary artery pathology such as a calcified aorta. For robotic-assisted approaches, careful attention to target anatomy is crucial since LAD exposure is limited through the minithoracotomy incision. This is less of a limitation for MICS CAB and TECAB approaches.
Chest wall anatomy is also an important consideration. Obese patients or female patients with large or pendulous breasts can make small-incision LIMA–LAD grafting challenging. Obesity can obscure landmarks, making port placement difficult, and may negate the potential benefits of a minimally invasive approach if LAD exposure is compromised. One exception to this is TECAB, which is less dependent on chest wall anatomy.
Contraindications
Hemodynamic instability, ongoing myocardial ischemia, and ventricular dysrhythmias are absolute contraindications for minimally invasive CABG. Patients must be hemodynamically stable and be able to tolerate single-lung ventilation, as well as the iatrogenic tension pneumothorax caused by intrathoracic carbon dioxide insufflation. Patients with previous thoracotomy, left chest radiation, or other thoracic trauma may develop dense adhesions that could preclude safe LIMA harvesting via the left chest. If unexpected adhesions are found upon insertion of the camera port, an attempt at adhesiolysis can be made, first using thoracoscopic instruments, and then the robotic arms; however, dense pleural adhesions usually preclude any further attempts at minimally invasive CABG. Other relative contraindications include untreated subclavian artery stenosis, severe chronic lung disease, and previous sternotomy. See Table 27.1 for a summary of patient characteristics to consider when contemplating robotic-assisted CABG or HCR.
For any minimally invasive cardiac operation, a skilled and dedicated surgical and anesthesia team are crucial for the success of the operation. For robotic procedures, there is even more emphasis on a skilled surgical team. For a significant portion of the procedure, the surgeon must rely on the assistant or surgical technician to manipulate
the robotic arms, exchange instruments, provide suture or clips, and troubleshoot problems. The circulating nurse must be able to troubleshoot technical difficulties that may arise with the robotic system, and the anesthesiologist must be able to manage hemodynamics and ventilation for patients with single-lung ventilation and carbon dioxide insufflation.
the robotic arms, exchange instruments, provide suture or clips, and troubleshoot problems. The circulating nurse must be able to troubleshoot technical difficulties that may arise with the robotic system, and the anesthesiologist must be able to manage hemodynamics and ventilation for patients with single-lung ventilation and carbon dioxide insufflation.
Surgical Options
MIDCAB
Minimally invasive direct coronary artery bypass grafting (MIDCAB) was introduced in the 1990s, but adoption of this technique faltered in the last decade due to concerns over suboptimal patency results, postthoracotomy pain from chest wall retraction, need for costal cartilage disarticulation, lung herniation, and incomplete LIMA harvesting. With the introduction of new retractor systems as well as increased experience with off-pump coronary artery bypass (OPCAB), MIDCAB has been a valuable option in several experienced centers. The LIMA is harvested under direct vision via a 6- to 8-cm anterolateral thoracotomy incision, and the anastomosis can be performed manually with either an off- or on-pump approach. Multivessel grafting is also an option with this approach and has been utilized by several centers. As described by Mcginn and Ruel for either single or multivessel grafting (MICS CABG), this requires a 4- to 7-cm thoracotomy in the fourth or fifth interspace and the use of a small rib-spreading retractor. Following pericardiotomy, target identification, and mammary harvest, proximal anastomoses are performed off the ascending aorta using a partial clamp.
EndoACAB
EndoACAB utilizes thoracoscopic access for LIMA harvest and pericardiotomy. This enables complete visualization of the LIMA and pericardiotomy, and target vessel identification, which then allows for a precise 3- to 5-cm anterolateral thoracotomy directly over the site of the anastomosis. The technique minimizes rib spreading and tissue trauma necessary for LIMA harvest associated with chest wall retraction during MIDCAB. The anastomosis is then performed with minimal, if any, rib spreading because the incision is tailored directly over the area of the planned anastomosis.
Robotic-Assisted CABG
Robotic technology has largely supplanted the endoscopic techniques of EndoACAB because of the superior exposure, three-dimensional and high-definition image quality and magnification, and improved range of motion afforded by the robotic instrumentation. This enables the surgeon to more easily navigate the contours of the chest wall during LIMA harvest. The LIMA harvest and pericardiotomy are performed in a similar fashion to EndoACAB, and the 3- to 4-cm incision can be tailored directly over the site of the anastomosis. Robotic-assisted CAB, or robotic MIDCAB, allows for a complete LIMA harvest, while the anastomosis is done manually through a limited anterior thoracotomy. This is our preferred approach and will be described in detail below.
TECAB
The most technically advanced of these procedures, with the longest learning curve, is TECAB. With this approach, robotic telemanipulation is extended to coronary grafting with the entire procedure performed endoscopically. Several specialized centers have reported excellent results with this technique, which can also be utilized for multivessel and multiarterial grafting strategies via a closed-chest approach. Encouraging results have been reported with both beating and arrested heart TECAB.
Cardiopulmonary Bypass
With each of these surgical strategies, surgeons can perform the anastomosis with or without cardiopulmonary bypass. Surgeon experience and comfort level dictate the preferred approach but the benefits of each must be balanced against the risks. Cardiopulmonary bypass enables the anastomosis to be performed without concerns for hemodynamic or electrical instability associated with coronary ischemia. LAD grafting is typically one of the easier anastomoses to perform off-pump due to its anterior location. For patients in whom distal ischemia is anticipated to be problematic, intracoronary shunts can allow for continuous perfusion during grafting. Furthermore, one of the benefits of off-pump LIMA–LAD grafting is the avoidance of any aortic manipulation, retrograde perfusion (with femoral cannulation), or the inflammatory sequelae of extracorporeal circulation, which may increase the risk of stroke or other complications.
Special Instruments/Equipment
All equipment required for a sternotomy CABG should either be on the surgical field or immediately available, including a functioning sternal saw and sternal retractor. The surgical team must be prepared to initiate cardiopulmonary bypass quickly, should the need arise. This requires the presence of a perfusionist, a bypass machine in the room, and all the necessary cannulas readily available. An endoscopic stabilizer is required, including the stabilizer arm that attaches to the OR (operating room) bed. Other essential routine OPCAB equipment include a carbon dioxide blower and silastic vessel loops. The LIMA harvest is accomplished using five robotic instruments: The cautery spatula, bipolar electrocautery, small and large clip appliers, and scissors. For the LIMA–LAD anastomosis, standard instruments for CABG surgery can be used, with the usual off-pump stabilizer being replaced by an endoscopic stabilizer (Octopus Nuvo, Medtronic Corporation, Minneapolis, MN). In addition, if a completion angiogram or a concomitant hybrid revascularization procedure is planned, then a hybrid OR is required.
Anesthesia Considerations
With regard to access and monitoring, a radial arterial line and central venous catheter often suffice. A pulmonary artery catheter and/or transesophageal echocardiography should be used in high risk patients, patients with left ventricular dysfunction, patients with valvular pathology, or early in one’s experience. A double-lumen endotracheal tube or endobronchial blocker provide single-lung ventilation and are left to the discretion of the attending anesthesiologist. One potential downside of the endobronchial blocker is that its use limits intermittent bilateral ventilation which may occasionally be necessary if ventilation becomes difficult or if procedure times are prolonged. Communication with the anesthesia team prior to carbon dioxide insufflation of the left chest is critical so that adequate blood pressure and intravascular volume can be maintained, mitigating the effects of an iatrogenic tension pneumothorax. Typically, if hypotension develops with carbon dioxide insufflation, it occurs quickly. Adequate volume loading and relative hypertension prior to insufflation will usually prevent this from occurring. If hypotension develops, the best course of action is to disconnect the insufflation and allow anesthesia the necessary time to make adjustments before reattempting insufflation. Insufflation pressures can also be decreased (8 to 10 mm Hg) to minimize the hemodynamic consequences of carbon dioxide insufflation.
Preparation and Positioning
A schematic of the OR setup is shown in Figure 27.1. It is important to be prepared and to practice for rare emergencies. Each participant in the surgical team must
understand their role, including performing several key steps such as bringing the bypass machine to the table and withdrawing the robotic arms and robot. Having the team run through mock “crashes” can be helpful to ensure efficiency during emergencies.
understand their role, including performing several key steps such as bringing the bypass machine to the table and withdrawing the robotic arms and robot. Having the team run through mock “crashes” can be helpful to ensure efficiency during emergencies.
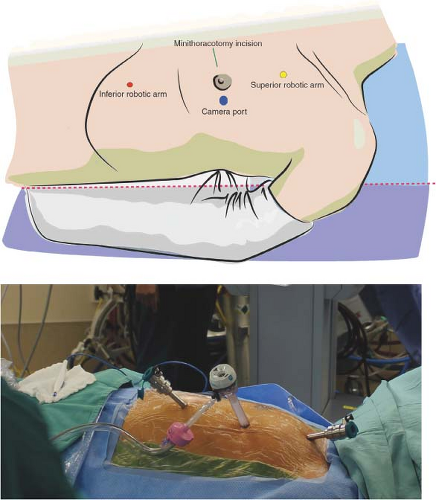
The patient is placed in supine position with a gel pad or shoulder roll placed inferior to left scapula to provide a 15-degree lift. The patient is positioned slightly to the left of the bed, allowing the left shoulder to fall slightly posteriorly off the edge of the bed, and the left arm is loosely tucked. This slight displacement of the left shoulder is necessary to allow maximum mobility of the right (superior) robotic arm (Fig. 27.2) and minimize conflict between this robotic arm and the left shoulder. The left arm should be protected with gel pads and forceful displacement of the left arm, which could result in brachial plexus injury, should be avoided. The thorax, groins, and legs are prepped in the standard fashion for traditional CABG; however, the field is extended to the left midaxillary line. In females, the left breast is pulled toward the midline and slightly caudad to decrease chest wall thickness during trocar insertion. The Ioban drape (3M, St Paul, MN) can help maintain this position. Following LIMA harvest, the breast should then be redraped and pulled further caudad or cephalad, depending on whether the minithoracotomy incision will be made through the superior portion of the breast, or along the inframammary crease. In women with larger breasts, we tend to avoid incisions in the inframammary crease, as they may be more susceptible to maceration and wound breakdown. The midsternum is marked to facilitate a midline incision in the event that conversion to sternotomy is necessary. Both groins remain exposed but covered with sterile drapes for femoral access in the event that angiography is required. The legs are prepped circumferentially in case venous conduit is needed. External defibrillator pads are positioned posteriorly on the left hemithorax and anteriorly over the right
pectoralis major and covered with clear nonporous sterile dressing to ensure that they remain dry and properly affixed following application of the sterile prep. The table is positioned with slight reverse Trendelenberg and slight right lateral rotation, which facilitates robotic LIMA harvest.
pectoralis major and covered with clear nonporous sterile dressing to ensure that they remain dry and properly affixed following application of the sterile prep. The table is positioned with slight reverse Trendelenberg and slight right lateral rotation, which facilitates robotic LIMA harvest.
Technique
Table 27.2 describes “pearls and pitfalls” of this technique.
Port Placement
The camera port (12 mm) should be placed at a location that provides optimal visualization of both the proximal and distal LIMA, and avoids obstruction from the heart while maintaining an adequate distance from the LIMA. Placement in the superior/inferior axis should be at the level of the midsternum (halfway between the sternal notch and beginning of the xiphoid process). The medial/lateral position of the camera port should be at the most acute angle of the ribs, usually approximately two fingerbreadths lateral to the midclavicular line (Fig. 27.2). A complementary method
for trocar positioning has been eloquently described by Sutter et al., referenced at the end of this chapter. Due to variations in individual chest wall anatomy, the surgeon must take into consideration the proximity of the camera port to the LIMA, the angle at which the port is placed, left ventricular size, and intrathoracic space. Adequate distance from the LIMA facilitates harvest; however, if the port is placed too far laterally, then the left ventricle may obscure visualization, and medial dissection of the LIMA can be more difficult. Prior to port placement, a 21-gauge needle attached to a 10-cm3



for trocar positioning has been eloquently described by Sutter et al., referenced at the end of this chapter. Due to variations in individual chest wall anatomy, the surgeon must take into consideration the proximity of the camera port to the LIMA, the angle at which the port is placed, left ventricular size, and intrathoracic space. Adequate distance from the LIMA facilitates harvest; however, if the port is placed too far laterally, then the left ventricle may obscure visualization, and medial dissection of the LIMA can be more difficult. Prior to port placement, a 21-gauge needle attached to a 10-cm3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree