INDICATIONS/CONTRAINDICATIONS
Many hybrid approaches have been reported in the pursuit of the ideal minimally invasive esophagectomy, but the main techniques include laparoscopic transhiatal esophagectomy, laparoscopic–thoracoscopic 3-hole (McKeown) esophagectomy, and laparoscopic—thoracoscopic (Ivor Lewis) esophagectomy. The approach is usually a matter of surgeon preference but on occasion is dictated by the location of the tumor. For the majority of distal esophageal tumors or gastroesophageal junction (GEJ) tumors, an Ivor Lewis approach allows good exposure and adequate margins. At the present time, we prefer the minimally invasive Ivor Lewis esophagectomy for most patients with esophageal adenocarcinoma, due to the predominant localization of esophageal adenocarcinoma at the GEJ or distal esophagus. Ivor Lewis MIE is also adequate for most esophageal squamous cell carcinomas in the mid- or distal esophagus. Ivor Lewis MIE may not be ideal for upper third or midesophageal cancers with significant proximal extension because resection with adequate margins may be difficult to obtain. In these cases, a modified McKeown MIE (3-hole) may be a good alternative. Esophagectomy may also be performed for patients with BE with HGD. In patients with HGD or early-stage tumors confined to the mucosa (T1a), satisfactory results have been reported with EMR with or without mucosal ablation (e.g. photodynamic therapy, radiofrequency ablation) when used under selected circumstances (e.g., well-circumscribed, well-differentiated tumors with no angiolymphatic invasion). It is important to note, however, that there have been only short- to intermediate-term outcomes reported thus far. In a well-known study, Ell et al.10 reported the updated results of EMR (with photodynamic therapy in 49%) for 100 highly selected patients with T1 intramucosal cancer (from 667 patients referred for early adenocarcinoma or HGD). The selection criteria for inclusion in their study included no angiolymphatic invasion, and histologic grades G1 and G2 (well differentiated and moderately differentiated, respectively) arising from BE. The lateral margins of resection were positive in 34% of patients, and could not be assessed in 33%. During a median 33-month follow-up, recurrent or metachronous lesions were detected in 11% of patients.10 While EMR in combination with ablation may offer several immediate benefits to the patient, there are several concerns.
1. Even in Ell’s experience,10 a large number of patients were screened and excluded from this approach, and only ideal candidates were chosen.
2. Even in experienced hands, positive margins are present in up to 34% of patients, with another 33% of patients with indeterminate margins due to cautery artifact, leaving only 33% with pathologically confirmed clear margins.10,11
3. Subsquamous BE remains at risk for progression to adenocarcinoma, and continued surveillance endoscopy is required.
4. When EMR is performed for early-stage tumors confined to the mucosa, incomplete nodal resection is a concern.
The rate of lymph node metastasis for T1a and T1b lesions is up to 7% and 27%, respectively,11 thus even when a negative margin is obtained in T1a tumors, a defined subset will fail this approach. Moreover, HGD is often multifocal and there is a high rate of occult carcinoma in patients who undergo resection for the preoperative diagnosis of HGD.12,13 Therefore, we continue to offer MIE for multifocal HGD and early-stage adenocarcinoma.
Patients with localized esophageal carcinoma and adequate cardiopulmonary reserve can be offered an MIE. Patients with locally advanced tumors and no distant metastasis on positron emission tomography (PET) scan who have a favorable response to neoadjuvant chemotherapy with or without concurrent radiation are also candidates for MIE.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
In the planning phase, detailed clinical and pathologic data should be obtained and reviewed by an experienced center. In our center, all patients undergo an upper endoscopy and a biopsy to confirm presence of esophageal cancer, its proximal and distal extent, and the suitability of stomach as a gastric conduit. Other preoperative studies include endoscopic ultrasonography (EUS) and positron emission tomography–computed tomography (PET—CT) scans for proper clinical staging. Many patients also require a stress test to assess underlying coronary artery disease. We perform pulmonary function tests on patients with a heavy smoking history or known chronic obstructive pulmonary disease to stratify their risk of respiratory complications. All patients undergo a bowel preparation the day before surgery. Subcutaneous heparin and intravenous antibiotics are administered on induction of general anesthesia.
 SURGERY
SURGERY
Patient Positioning
 A double-lumen endotracheal tube is placed and its location is confirmed with a pediatric endoscope. The bronchial cuff (blue) is deflated during the abdominal portion of the procedure to minimize the risk of left main stem ischemic injury. If the tumor is localized to the upper esophagus or midesophagus, we routinely perform an initial bronchoscopy with a single-lumen endotracheal tube.
A double-lumen endotracheal tube is placed and its location is confirmed with a pediatric endoscope. The bronchial cuff (blue) is deflated during the abdominal portion of the procedure to minimize the risk of left main stem ischemic injury. If the tumor is localized to the upper esophagus or midesophagus, we routinely perform an initial bronchoscopy with a single-lumen endotracheal tube.
 We start the operation by performing an on-table endoscopy to confirm preoperative findings and ensure that the stomach is suitable as a gastric conduit. This defines the exact location of tumor and proximal extent of BE.
We start the operation by performing an on-table endoscopy to confirm preoperative findings and ensure that the stomach is suitable as a gastric conduit. This defines the exact location of tumor and proximal extent of BE.
 The patient is placed supine and positioned slightly toward the right edge of the table. A footboard is placed to prevent the patient from slipping during steep reverse Trendelenburg positioning.
The patient is placed supine and positioned slightly toward the right edge of the table. A footboard is placed to prevent the patient from slipping during steep reverse Trendelenburg positioning.
Laparoscopic Phase
Port Placement/Staging
 The surgeon operates from the right side of the table and the assistant is on the left side. Six abdominal ports are placed, five as depicted in Figure 24.1 and a right lower costal margin port for the Mediflex liver retractor (Mediflex surgical products, Islandia, NY). The peritoneal surface, omentum and the liver are thoroughly inspected during the initial laparoscopic staging to rule out any occult metastasis. If there is a suspicion for liver metastasis on preoperative imaging, an intraoperative liver ultrasound may be performed. The suitability of the stomach for use as a conduit is also assessed laparoscopically.
The surgeon operates from the right side of the table and the assistant is on the left side. Six abdominal ports are placed, five as depicted in Figure 24.1 and a right lower costal margin port for the Mediflex liver retractor (Mediflex surgical products, Islandia, NY). The peritoneal surface, omentum and the liver are thoroughly inspected during the initial laparoscopic staging to rule out any occult metastasis. If there is a suspicion for liver metastasis on preoperative imaging, an intraoperative liver ultrasound may be performed. The suitability of the stomach for use as a conduit is also assessed laparoscopically.
 The patient is placed in a steep reverse Trendelenburg position and the abdomen is insufflated using 10 to 15 mm Hg carbon dioxide insufflation.
The patient is placed in a steep reverse Trendelenburg position and the abdomen is insufflated using 10 to 15 mm Hg carbon dioxide insufflation.
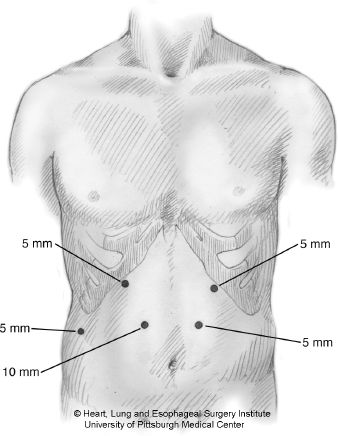
Figure 24.1 Laparoscopic port placement. The 10-mm port is placed first in the right midabdomen using open Hasson trocar insertion technique. An additional 5/11-mm port (not shown) is placed in the right lower quadrant that is helpful for retraction during pyloroplasty and gastric tube creation.
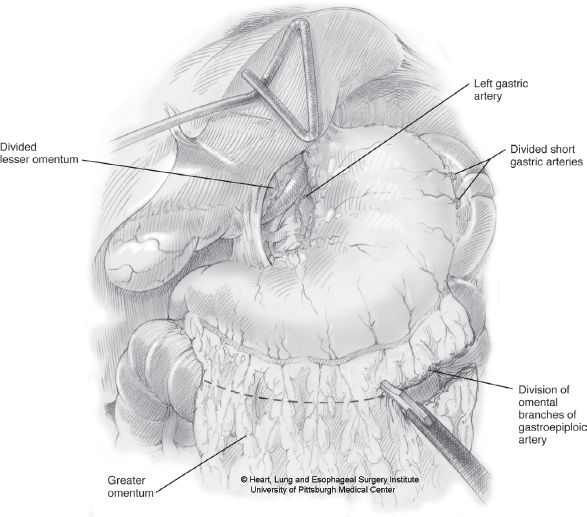
Figure 24.2 Laparoscopic staging, with opening of the gastrohepatic ligament and evaluation of left gastric/celiac lymph nodes.
 Next, the gastrohepatic ligament is opened using ultrasonic energy. Dissection is carried along the left gastric vessels. A formal left gastric and celiac lymph node dissection is done, and any suspicious nodes are dissected and sent for frozen-section analysis (Fig. 24.2).
Next, the gastrohepatic ligament is opened using ultrasonic energy. Dissection is carried along the left gastric vessels. A formal left gastric and celiac lymph node dissection is done, and any suspicious nodes are dissected and sent for frozen-section analysis (Fig. 24.2).
 Once assured that minimal nodal disease is present and the tumor appears completely resectable, then we proceed to crural dissection and complete mobilization of the lower esophagus.
Once assured that minimal nodal disease is present and the tumor appears completely resectable, then we proceed to crural dissection and complete mobilization of the lower esophagus.
 Esophageal mobilization is performed at the level of the diaphragmatic crura. The right crus is separated from the esophagus; dissection is carried anteriorly and cephalad to free the left crus and the gastric fundus (Figs. 24.3 and 24.4). The esophagus is mobilized circumferentially at the hiatus from the preaortic tissue plane posteriorly to the pericardium anteriorly and from right to left pleura.
Esophageal mobilization is performed at the level of the diaphragmatic crura. The right crus is separated from the esophagus; dissection is carried anteriorly and cephalad to free the left crus and the gastric fundus (Figs. 24.3 and 24.4). The esophagus is mobilized circumferentially at the hiatus from the preaortic tissue plane posteriorly to the pericardium anteriorly and from right to left pleura.
 At this point, provided there is no metastatic disease, bulky adenopathy, or evidence of a T4 tumor, one can proceed with esophagectomy.
At this point, provided there is no metastatic disease, bulky adenopathy, or evidence of a T4 tumor, one can proceed with esophagectomy.
 If at this point, a decision is made to delay the MIE and give neoadjuvant therapy, consideration can be given to some vascular conditioning, such as dividing the left gastric artery and vein and the short gastrics. However, we have noted when operation is delayed and a significant amount of periesophageal and gastric dissection have been done, this can lead to significant dense scar tissue and adhesions at the time of definitive resection.
If at this point, a decision is made to delay the MIE and give neoadjuvant therapy, consideration can be given to some vascular conditioning, such as dividing the left gastric artery and vein and the short gastrics. However, we have noted when operation is delayed and a significant amount of periesophageal and gastric dissection have been done, this can lead to significant dense scar tissue and adhesions at the time of definitive resection.
Mobilization of the Stomach
 The medial border of the right crus is dissected inferiorly toward the decussation of the crura. A retroesophageal window is created. Additional mobilization of the lesser curve along the left gastric vascular pedicle is performed.
The medial border of the right crus is dissected inferiorly toward the decussation of the crura. A retroesophageal window is created. Additional mobilization of the lesser curve along the left gastric vascular pedicle is performed.
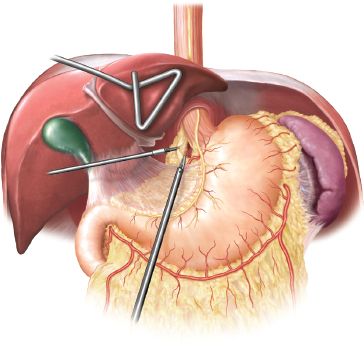
Figure 24.3 Laparoscopic staging (continued). The right crus is separated from the esophageal wall/fat pad and the decussation of the crura is identified. Resectability along these tissue planes and along the left crus is confirmed before proceeding to left gastric vascular pedicle ligation and division.
 The superior portion of the greater curve is mobilized, starting at the level where the last gastroepiploic arcade vessel enters the gastric wall, and proceeds cephalad along the greater curve. Care is taken to avoid direct handling of the conduit portion of the stomach. We try to grasp areas that will be resected as opposed to handling the neoesophagus proper. Short gastric vessels are divided up to the left crus. We use autosonic shears or the Ligasure device (Covidien Surgical, Mansfield, MA).
The superior portion of the greater curve is mobilized, starting at the level where the last gastroepiploic arcade vessel enters the gastric wall, and proceeds cephalad along the greater curve. Care is taken to avoid direct handling of the conduit portion of the stomach. We try to grasp areas that will be resected as opposed to handling the neoesophagus proper. Short gastric vessels are divided up to the left crus. We use autosonic shears or the Ligasure device (Covidien Surgical, Mansfield, MA).
 The lesser sac is entered below the gastric antrum, preserving the right gastroepiploic arcade. The attachments to the transverse colon are mobilized to fully free the greater curve. This completes the antropyloric mobilization.
The lesser sac is entered below the gastric antrum, preserving the right gastroepiploic arcade. The attachments to the transverse colon are mobilized to fully free the greater curve. This completes the antropyloric mobilization.
 The fundus is rotated toward the patient’s right side and all retrogastric attachments in the lesser sac are taken down toward the lesser curve until the left gastric vessels are seen. This retrogastric dissection is carried posteriorly toward the pylorus. The degree of Kocher maneuver that is needed is up to the judgment of the surgeon. In general, we free up the antrum and pylorus and free the first portion of the duodenum from the gall bladder. Once the pylorus is mobilized enough to be lifted and reach the right crus without any tension, the mobilization is usually sufficient.
The fundus is rotated toward the patient’s right side and all retrogastric attachments in the lesser sac are taken down toward the lesser curve until the left gastric vessels are seen. This retrogastric dissection is carried posteriorly toward the pylorus. The degree of Kocher maneuver that is needed is up to the judgment of the surgeon. In general, we free up the antrum and pylorus and free the first portion of the duodenum from the gall bladder. Once the pylorus is mobilized enough to be lifted and reach the right crus without any tension, the mobilization is usually sufficient.
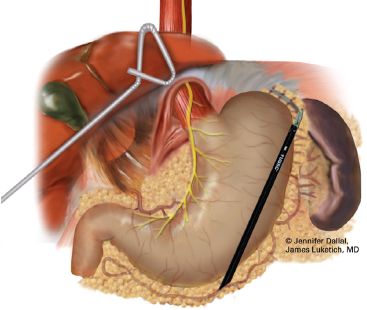
Figure 24.4 Division of highest short gastric vessels and dissection along the greater curve of the stomach.
 The left gastric vessels are skeletonized and divided at the take off from the celiac artery with a vascular load of the Endo GIA stapler. Care is taken to maximize lymph node yield by sweeping all lymph nodes and fatty tissue up to the specimen side.
The left gastric vessels are skeletonized and divided at the take off from the celiac artery with a vascular load of the Endo GIA stapler. Care is taken to maximize lymph node yield by sweeping all lymph nodes and fatty tissue up to the specimen side.
Creation of Gastric Conduit
 The first assistant stretches the gastric fundus by holding it along the line of the short gastrics and gently pulling it above the upper pole of the spleen. The gastric antrum is pulled toward the right lower quadrant from the additional 5/11-mm port (Fig. 24.5). This stretches the stomach to some degree, leading to a longer conduit as staple loads are applied and minimizes the risk of spiralling of the gastric tube.
The first assistant stretches the gastric fundus by holding it along the line of the short gastrics and gently pulling it above the upper pole of the spleen. The gastric antrum is pulled toward the right lower quadrant from the additional 5/11-mm port (Fig. 24.5). This stretches the stomach to some degree, leading to a longer conduit as staple loads are applied and minimizes the risk of spiralling of the gastric tube.
 The vascular tissue along the lesser curve is divided above the level of the right gastric artery with a vascular load (tan load, three rows of staples [tri-staple technology] of height 2 mm, 2.5 mm, and 3 mm) of an Endo GIA stapler (Covidien Surgical). Next, sequential firing of Endo GIA purple loads (three rows of staples; height 3 mm, 3.5 mm, 4 mm) is performed across the antrum and toward the fundus trying to keep the staple line parallel to the line of the short gastrics with a distance of 2.5 to 3 cm between the staple line and the short gastric vessels.
The vascular tissue along the lesser curve is divided above the level of the right gastric artery with a vascular load (tan load, three rows of staples [tri-staple technology] of height 2 mm, 2.5 mm, and 3 mm) of an Endo GIA stapler (Covidien Surgical). Next, sequential firing of Endo GIA purple loads (three rows of staples; height 3 mm, 3.5 mm, 4 mm) is performed across the antrum and toward the fundus trying to keep the staple line parallel to the line of the short gastrics with a distance of 2.5 to 3 cm between the staple line and the short gastric vessels.
 An unusually thick antrum may require that one chooses a greater staple height (e.g., the black Endo GIA loads, staple height 4 mm, 4.5 mm, 5 mm) to get an adequate staple line integrity. Generally, even when there is a thick antrum, the final stapler firings across the thinner fundus can be reduced to the purple loads with 3 mm, 3.5 mm, and 4 mm tri-staple heights (Fig. 24.6).
An unusually thick antrum may require that one chooses a greater staple height (e.g., the black Endo GIA loads, staple height 4 mm, 4.5 mm, 5 mm) to get an adequate staple line integrity. Generally, even when there is a thick antrum, the final stapler firings across the thinner fundus can be reduced to the purple loads with 3 mm, 3.5 mm, and 4 mm tri-staple heights (Fig. 24.6).
 The gastric tube is generally created before completion of other abominal steps to provide time to assess the viability of the gastric tube as a conduit before bringing it into the chest.
The gastric tube is generally created before completion of other abominal steps to provide time to assess the viability of the gastric tube as a conduit before bringing it into the chest.
 Recently, we have added an omental flap on all cases where preoperative radiation therapy was used.14 This can be performed by sparing two or three of the omental arcades that leave the gastroepiploic arcade in a perpendicular fashion. We then carefully follow these arcades out toward the free omentum for a distance of 10 cm or more, thus creating 2- to 3-cm wide, 10 cm or more long vascularized omental pedicle (Fig. 24.7).
Recently, we have added an omental flap on all cases where preoperative radiation therapy was used.14 This can be performed by sparing two or three of the omental arcades that leave the gastroepiploic arcade in a perpendicular fashion. We then carefully follow these arcades out toward the free omentum for a distance of 10 cm or more, thus creating 2- to 3-cm wide, 10 cm or more long vascularized omental pedicle (Fig. 24.7).
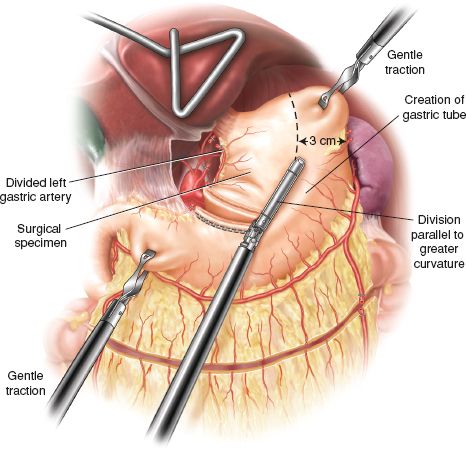
Figure 24.5 Creation of the gastric conduit. The first stapler along the lesser curve is a vascular Endo GIA stapler after which the thick antrum is divided as described in the text. The antrum and the fundus are pulled in opposite directions to provide adequate tension during the gastric conduit creation.



