17 Metastatic Tumors in the Lung
A Practical Approach to Diagnosis
The most common form of pulmonary neoplasm is a metastasis from outside the lungs. Based on autopsy data, the lungs are involved by metastatic lesions in 25% to 55% of malignant diseases,1–5 and, in up to one fourth of those cases, the pulmonary parenchyma and pleura are the only sites of distant spread.4 On the other hand, the most common lung tumor encountered by a practicing surgical pathologist or cytopathologist is primary bronchogenic carcinoma; distinguishing primary from secondary pulmonary neoplasms is a major challenge. This chapter provides a concise background discussion of the pathobiologic principles and clinicoradiologic findings of metastases in the lungs and offers a framework for the practitioner to identify such lesions with an optimal level of certainty.
Routes of Spread for Intrapulmonary Metastases
Vascular Metastases
Most metastatic tumors in the lungs have arrived at that destination hematogenously. There are two principal reasons for this: The lungs receive the entire cardiac output, and they contain a rich vascular network, comprising a huge capillary bed. The detailed principles underlying vascular metastasis have been outlined elsewhere.6–8 Malignant tumors may contain subclones9 of cells with differing metastatic potential, and some of them acquire the ability to enter the bloodstream as microemboli. At selected distant sites, they adhere to endothelial basement membranes, and through a process known as extravasation, the tumor cells move through the extracellular matrix to form metastatic deposits in various parenchymal structures. In this paradigm, the original micrometastasis then proliferates to yield a larger mass, which later (often as long as several years) may become visible clinically or radiographically. Most pulmonary metastases show nests of neoplastic cells that are surrounded by, and intercalated with, a variable quantity of fibrous stroma. At this stage, no cells typically remain inside the pulmonary arterial, venous, or capillary system.
The rate at which potentially metastasizing cellular subclones develop (if they do at all) in primary tumors varies considerably; the probability that such lesions will spread hematogenously depends on both tumor-related factors and conditions in the milieu of the host tissues. For reasons that are largely unknown, some tumors—such as osteosarcoma—often shed micrometastases before the primary tumor is detected clinically; other tumors may show metastasis only very late in their biologic evolution.10
The clinical presentation of patients with hematogenous pulmonary metastases is variable. Most patients have no symptoms,11 and their lesions are detected only through imaging studies that are undertaken for staging purposes or for surveillance during treatment. The radiographic appearance of metastatic pulmonary lesions may be that of a single central or peripheral mass, multiple central or peripheral masses, diffuse infiltrates, or a combination of the latter two possibilities. If a patient is symptomatic, the clinical findings reflect the location and extent of the metastatic deposits and commonly include chest pain, dyspnea, cough, hemoptysis, and wheezing, to name a few.
In addition to the concept of microembolization as outlined earlier, tumors may also spread as macroscopic emboli that involve large or medium pulmonary arteries.12 Large-vessel tumor emboli may cause acute heart failure, sudden death, rapidly evolving pulmonary hypertension, and pulmonary infarction, as also seen with banal intravascular thromboemboli associated with deep venous thrombosis.13–15 The tumors that most commonly give rise to macroscopic emboli are those associated with major systemic veins (e.g., renal or hepatic carcinomas invading the renal and hepatic veins) and primary tumors of the heart (myxomas and sarcomas).16
In rare cases, hematogenous metastasis principally occurs in the lung with occlusive luminal tumor plugs in small vessels (arterioles and capillaries) without interstitial involvement or formation of masses.17 In a sense, those tumors have not gone through all of the biologic steps normally associated with the metastatic sequence, but they are nonetheless potentially lethal because they may cause severe pulmonary hypertension. Neoplasms that may show that pattern of spread (sometimes called tumor-related thrombotic pulmonary microangiopathy18) include carcinomas of the breast, gastrointestinal tract, liver, pancreas, uterus, gallbladder, prostate, and ovary.17 Soares et al. reported that most malignant tumors that occlude small pulmonary vascular channels also can be seen simultaneously within larger vessels.19 Patients with metastatic microvascular occlusion typically present with progressive dyspnea and cor pulmonale.17,18
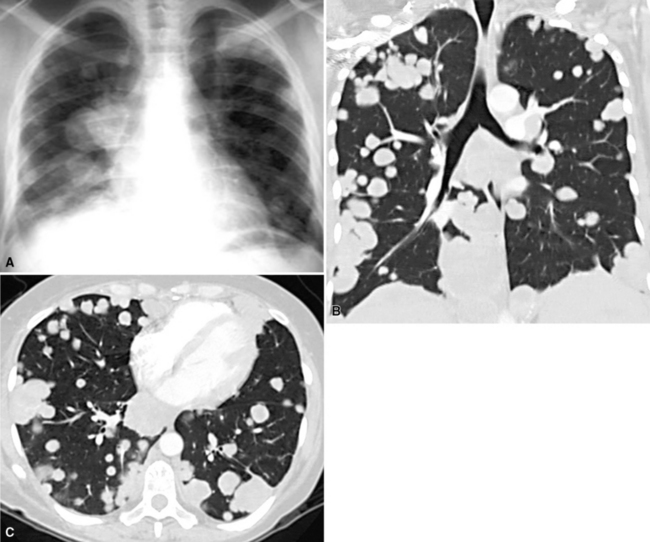
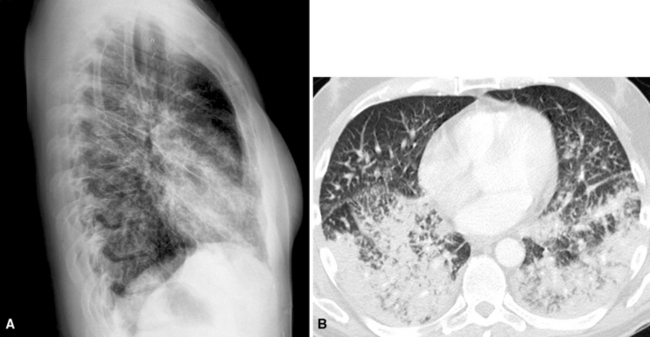
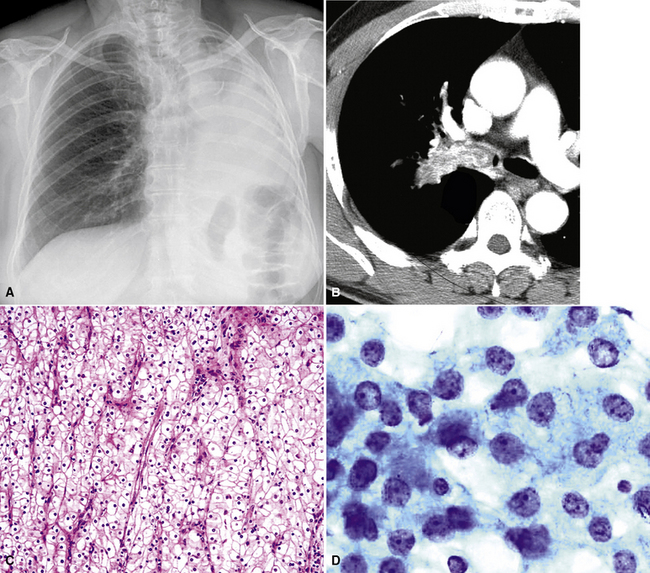
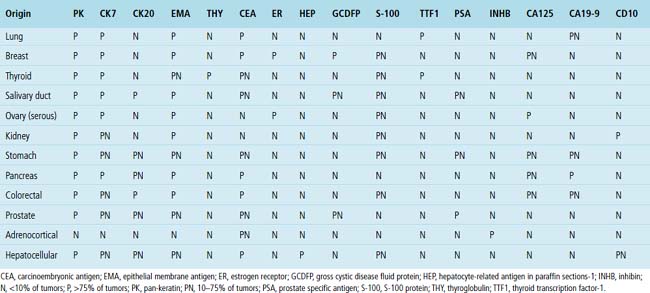
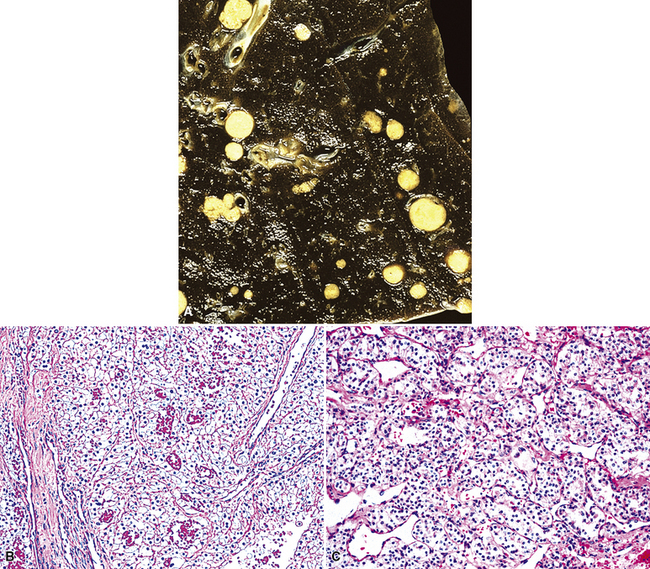
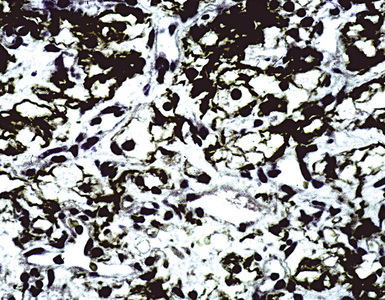
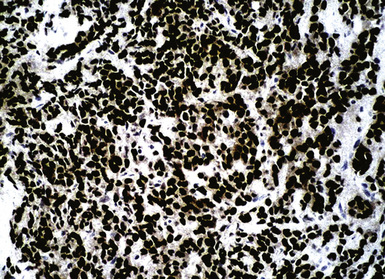
Hematogenous metastasis in the lung is associated with a spectrum of radiologic findings. The most common roentgenographic appearance is that of multiple, bilateral, variably sized masses (Figs. 17-1 to 17-3); occasionally, a solitary intraparenchymal nodule is seen. The metastatic implants usually appear in the mid- to lower lung field because that is where the greatest parenchymal perfusion occurs. In up to 90% of patients with bilateral secondary disease, the lesions are peripheral and subpleural (Fig. 17-4).1,20 Variability in the size of the metastatic nodules is related to the different “ages” of the lesions, dissimilar growth rates, and other factors. Such lesions are usually smaller than primary pulmonary carcinomas, measuring less than 3 to 4 cm in maximum diameter. Metastases also enlarge more rapidly than bronchogenic carcinomas do.
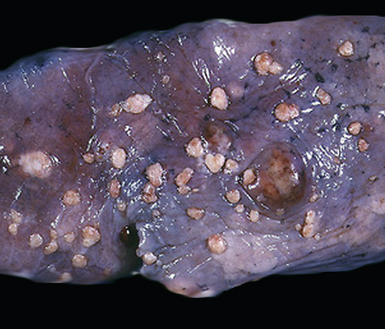
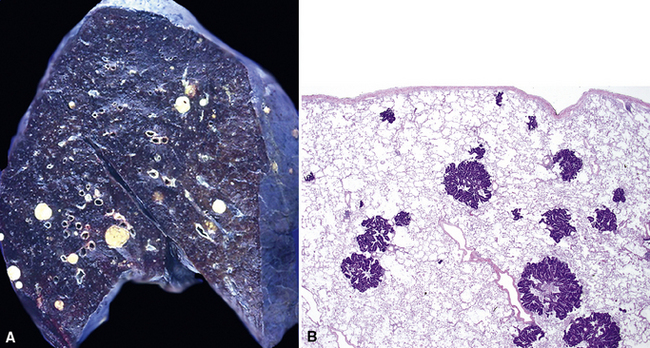
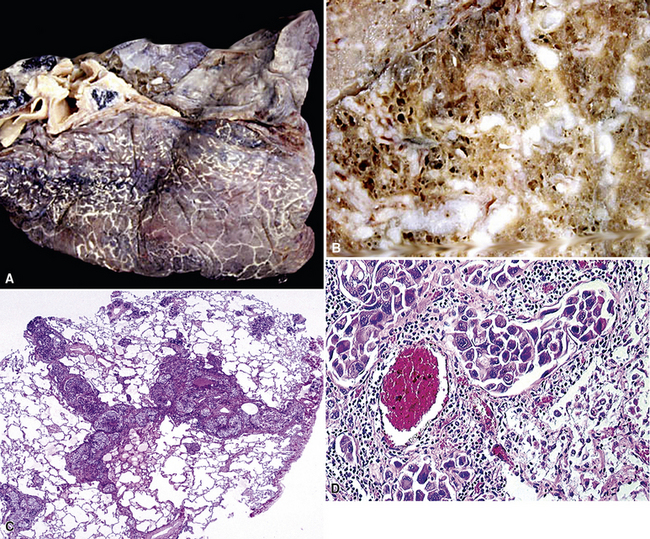
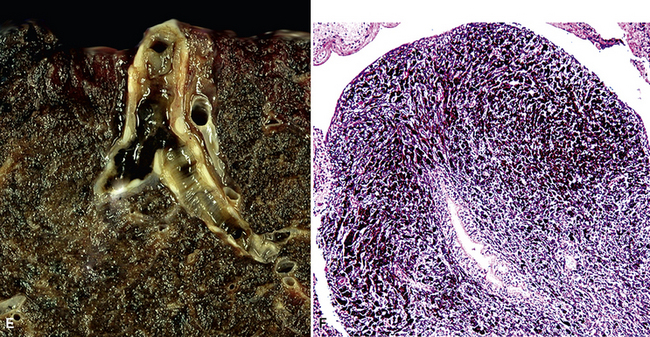
Figure 17-2 Gross photograph of metastatic carcinoma in the lungs showing several subpleural nodules of varying size.
Solitary metastases in the lungs are seen in up to 10% of all malignant tumors involving those organs.21–23 Filderman and coworkers suggested that solitary lesions larger than 5 cm in diameter most likely originate in the breast, kidney, or soft tissue.6 Metastasis in the form of a solitary mass on plain films may appear as multiple contiguous or coalescent masses on computed tomograms. Quint and colleagues reported that, statistically, patients with carcinomas of the head and neck, bladder, breast, cervix, bile ducts, esophagus, ovary, prostate, or stomach were more likely to have a solitary metastasis to the lung than a new primary bronchogenic tumor, even when a significant period had elapsed after diagnosis of the extrapulmonary neoplasms.24 On the other hand, patients with a history of malignant melanoma, sarcoma, or malignant germ cell tumor were more likely to have a second primary malignancy of the lung under the same circumstances.24
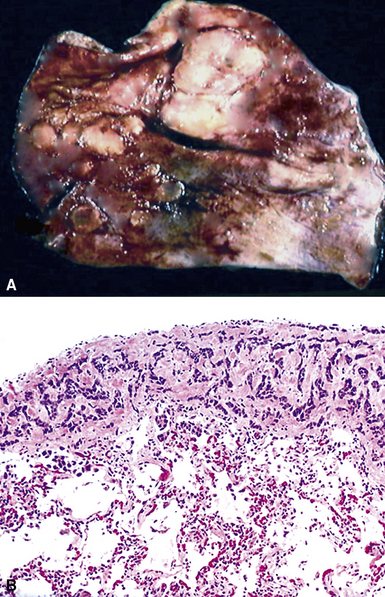
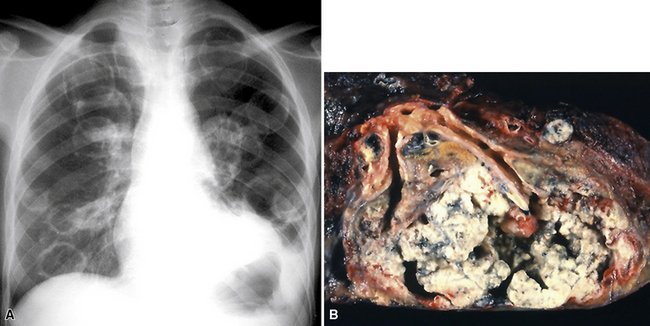
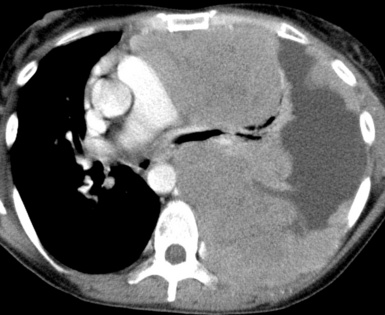
Large metastatic foci may undergo cavitation or result in pneumothorax or bronchopleural fistulization (Fig. 17-5). The most common secondary tumor that cavitates is squamous cell carcinoma, often originating in the head and neck.25 Metastatic sarcoma and adenocarcinoma also may exhibit that feature.25 Pneumothoraces and transpleural fistulae result from erosion by the metastatic tumor through the pleura, as seen most frequently in pediatric mesenchymal malignancies (e.g., osteosarcoma).26
Lymphogenous Metastases
One study27 reported that up to 56% of pulmonary metastases were lymphogenously mediated, although a more generally accepted figure is 5% to 8%.28,29 Most patients with lymphatic-borne lung metastases have a poor prognosis, with 90% dying within 6 months.29
The radiographic appearance of lymphangitic metastasis is variable; in 50% of cases, plain chest films show no apparent abnormality.30 Yang and Lin described four radiologic patterns for this condition29:
With the first two patterns representing secondary tumors, pleural, or less frequently, hilar lymph nodal involvement may be seen. More than 90% of these cases are metastatic adenocarcinomas.29 It is estimated that fewer than 1% of patients with pulmonary metastases from tumors arising outside the thorax also have hilar adenopathy.31 Although this figure may appear low, hilar adenopathy in patients with secondary pulmonary malignancies is not uncommon in absolute terms because of the high prevalence of patients with metastatic disease.
Carcinomas may gain access to the pulmonary lymphatic system by retrograde spread, direct invasion of the pulmonary lymphatics, and passage through adjacent blood vessels. The most common route of spread is the last of these three possibilities32; tumor first spreads hematogenously to the lung and results in small areas of interstitial growth. The neoplastic cells are then absorbed into the lymphatics and permeate further throughout the lungs (Fig. 17-7). Tumor in alveolar spaces may likewise be absorbed through the lymphatics adjacent to terminal bronchioles. Therefore, patients who have lymphangitic intrapulmonary metastases generally also have had previous hematogenous spread.32 Direct lymphatic invasion is most often associated with tumors arising in the breast or stomach29,33,34; in this mode, metastases may be seen exclusively in the pulmonary lymphatic spaces, without the formation of mass lesions. Other metastatic carcinomas capable of showing the same pattern of spread are those arising in the ovary, thyroid, bladder, esophagus, and liver.29,32
Pleural Metastases
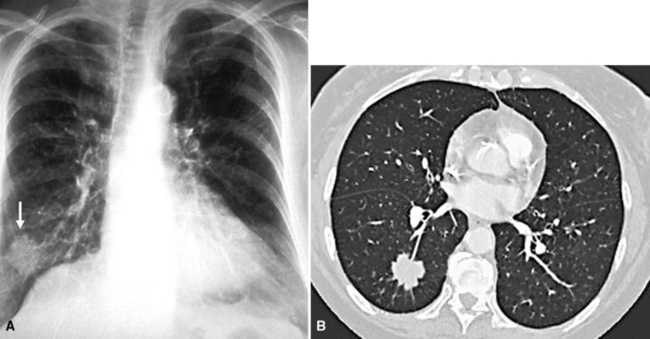
Metastases are the most common form of pleural malignancy. Most derive from primary neoplasms of the chest wall, mediastinum, or lungs,6 although extrathoracic primary malignancies are also well represented. The largest tumor nodules tend to be basally situated in the chest35 (Fig. 17-8).
At least two thirds of malignant pleural effusions can be diagnosed by cytologic sampling of the pleural fluid. More than 90% of cases are recognized on the first specimen,36,37 but sensitivity predictably increases with successive sampling; three specimens are routinely recommended if the clinical suspicion of pleural metastasis is high.38
Malignancy is second only to congestive heart failure as a cause of pleural effusions.39 Neoplastic effusions are typically described as “massive” or “copious,” ranging up to 2500 mL in volume,40 and they are often bloody. Nevertheless, malignant involvement of the pleura may also be associated with scant fluid production and a serous character.35 Obviously, not all pleural effusions in patients with a history of malignancy contain tumor cells41; benign effusions in such cases may be secondary to lymphatic obstruction, altered lymphatic drainage as a result of chemotherapy or radiation therapy,42 heart failure, or other causes. Because most sarcomas do not spread via lymphatic channels, metastases in the lung and pleura are not usually accompanied by a tumorous effusion.43 Up to 90% of malignant pleural effusions caused by metastatic lung or breast cancers are ipsilateral with regard to the site of the original tumor.35 Patients with malignant pleural effusions typically have a dismal prognosis, and most die within a few months of diagnosis.44–46 Selected subgroups of patients, such as those with lymphoma, breast cancer, or some pediatric malignancies, may fare somewhat better.
Chretien and Jaubert reported that 42% of cytologically sampled pleural effusions contained malignant cells.47 The likely site of tumor origin in such cases appears to depend on patient demographics, although most series have reported that primary pulmonary carcinomas are the most common.45,48 Squamous cell carcinomas of the lung do not usually involve the pleural fluid; however, adenocarcinomas usually do (Fig. 17-9), followed by small cell neuroendocrine carcinoma.49,50 Almost any other extrathoracic malignancy may metastasize to the pleural space, but the most common tumors that do so are carcinomas arising in the breast, gastrointestinal tract, and ovary; non-Hodgkin lymphoma is also well represented.45,48,51 Up to 7% of metastatic carcinomas in the pleura must be classified as originating in an unknown primary location.48 In one analysis of malignant pleural effusions, women predominated by a ratio of 2:1,40 but no sex preferences were seen in other series.45
Endobronchial Metastases
Endobronchial metastases are considered in a separate category because of their distinctive clinical findings, principally the syndrome of “adult-onset asthma.” The reported incidence of endobronchial and endotracheal metastatic disease is 1% to 18% of patients who also have intrapulmonary metastases.11,52,53 The most common sites of tumor origin in patients from North America and Western Europe are the breast, bone, soft tissue, large intestine, kidney, and skin (melanoma).11,54–58 More than one third of endobronchial metastases are sarcomatous.53,57 In populations with a high prevalence of acquired immune deficiency syndrome, the most common secondary malignancies of the bronchi are Kaposi sarcoma and malignant lymphoma.59 Nasopharyngeal and laryngeal carcinomas are frequent sources of endobronchial metastasis in Asia.60
Endobronchial metastases may be either hematogenous or lymphogenous.11,53,57 Aerogenous spread of an upper-airway malignancy has also been suggested as a possibility. Tumors that originate in the lung, hilar lymph nodes, or mediastinum may spread by direct extension into the bronchial system. Endobronchial lesions cause symptoms early in their course of growth, namely, cough with sputum production, dyspnea, wheezing, infection, and hemoptysis.60,61 However, up to 25% of patients are asymptomatic.58 Radiographically, an endobronchial mass is typically visible only on computed tomography or magnetic resonance scan; plain film studies commonly show only postobstructive consolidation or atelectasis (Fig. 17-10). The mean interval between diagnosis of the original tumor and the appearance of endobronchial metastasis is 4 to 5 years.61 These patients have a poor survival, with a median of 11 months61; patients with breast cancer may have a better prognosis.54,57,58
Modalities for the Diagnosis of Pleuropulmonary Metastases
The diagnostic tests that are typically used for any suspected pleuropulmonary tumor are also applicable to the study of metastatic disease. These include sputum cytology,62,63 bronchoscopy with brushing, washing, alveolar lavage, transbronchial or transtracheal aspiration and biopsy,64–66 transthoracic fine-needle aspiration (FNA),67,68 thoracoscopic biopsy (video-assisted thoracic surgery),69–71 open thoracotomy and biopsy, and effusion cytology.46 In some cases, expectant management is undertaken, with sequential radiologic studies.72,73 Computed tomography scans may be obtained to further delineate abnormalities and possibly to aid in the distinction between primary and secondary malignancies; for example, mediastinal adenopathy favors a primary lung tumor.74 When multiple radiographic lesions of the lung or pleura are detected radiographically in some patients with well-documented histories of malignancy, further diagnostic evaluations may be eschewed.
For the most part, the diagnostic accuracy of tests that yield tissue for pathologic examination generally has not been determined in this specific context. The accuracy is believed to depend principally on the size and location of the lesion rather than its specific histologic nature. For example, the sensitivity of FNA is 93% if the lesion is larger than 2 cm in diameter and 60% for those smaller than 1 cm. Higher sensitivity is realized in the sampling of peripheral nodules compared with central lesions, regardless of whether they are primary or secondary.75 Pilotti et al. reported that the sensitivity of FNA in the detection of metastatic pleuropulmonary disease was 89%, whereas it was 92% for primary malignancies.76 Another interinstitutional study reported 96% specificity for transthoracic FNA.77 The sensitivity of bronchoscopy also depends on the location of the lesion; as expected, that technique is particularly well suited for the visualization and sampling of endobronchial metastases.64–66 Thoracoscopy is a sensitive means of accessing peripheral lesions and has a high level of accuracy overall. It may be viewed as a treatment option as well as a diagnostic test in patients who have limited metastatic disease, especially if lung function is compromised.69–71
Kern and Schweizer concluded that the sensitivity of sputum cytology for the detection of intrapulmonary metastasis was similar to that associated with primary lung cancers.78 However, that technique is much more likely to be productive if the metastatic lesions are large and centrally located.78
Practical Approach to Differential Diagnosis
A definite challenge in pulmonary pathology is determining whether a newly detected lung mass is primary or secondary in patients with or without a history of extrapulmonary malignancy. If no previous tumor has been seen and the lesion has the morphologic attributes of a nonpulmonary proliferation, it is necessary to search for a primary site. Malignancies that are clinically occult and present with pulmonary metastases are not unusual, and they account for approximately 2% to 5% of all metastatic carcinomas of unknown origin (MCUOs).79,80 Because of the treatment-related and prognostic issues concerning secondary malignancies of the lung, it may be decided that additional resources are not justified to determine the primary location of the tumor.81
With regard to the general distinction of primary and metastatic pulmonary tumors, one generally depends on radiographic findings, histologic features, microscopic comparison of the current lesion with any previous malignancies, and the use of ancillary pathologic studies, such as immunohistochemistry, cytochemistry, molecular biologic techniques, cytogenetic methods,82 and electron microscopy. If paraffin-embedded tissue from previous tumor material is available, immunopathologic studies of the previous tumor and the current specimen can be obtained comparatively.
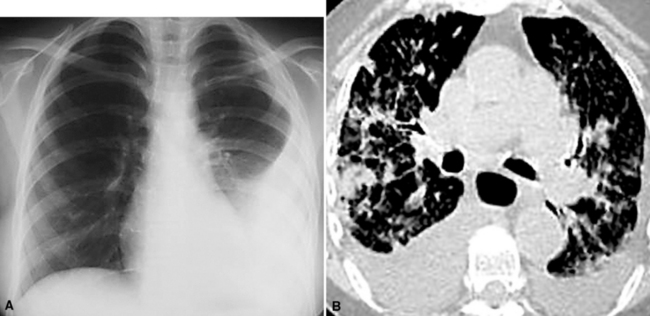
Useful information for the distinction between primary and secondary neoplasms in the lung may be derived from details of the clinical evaluation and physical findings.83,84 For example, the characteristically “spiculated” appearance of primary lung cancers (Fig. 17-11) on imaging studies of the chest distinguishes them from the more rounded and well-delimited appearance of metastases. Unfortunately, such information is often not made available to pathologists, even though it is well known to enhance diagnostic accuracy.85 A high index of suspicion must be maintained, and communication with the radiologist should occur whenever the pathologist has increased concern, histologic or otherwise, that a tumor may be a metastasis. Open communication is essential if the patient has a history of oncologic disease.
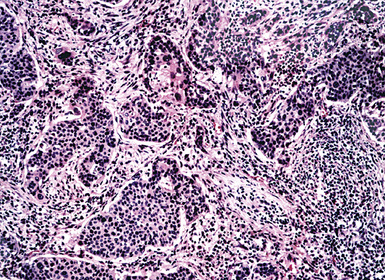
The light microscopic features of any given lesion are the cornerstone of pathologic diagnosis. The appearance of the lesion after hematoxylin and eosin staining is often sufficient to determine whether the tumor is primary or metastatic. Carcinomas arising in the lung typically have evolved over several years before coming to clinical attention. As a consequence, the host responds to such lesions by surrounding them with an irregular cuff of fibroinflammatory tissue (Fig. 17-12). The mixture of proliferation and degeneration that characterizes primary carcinomas commonly causes central zones of fibrosis, with entrapment of some residual native pulmonary structures. In contrast, metastases to the lung parenchyma have a “clean” interface with the surrounding tissue and are not associated with peripheral zones of fibroinflammatory response. Because they are rapidly growing vis-à-vis bronchogenic neoplasms, metastatic carcinomas also lack centrally sclerotic regions. These “rules” do not apply universally to all tumor types. Specifically, primary and metastatic sarcomas, adenocarcinomas with a “lepidic” growth pattern, and small cell neuroendocrine carcinomas are essentially superimposable morphologically.
Adenocarcinoma Variants
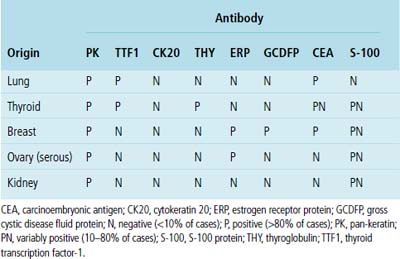
Adenocarcinoma is the most common form of primary lung cancer. In patients who have a history of an extrapulmonary adenocarcinoma, the distinction between a primary and secondary lesion may be challenging. In some cases, morphologic appearances alone are sufficient to accomplish that task, as discussed later. Immunopathology is also helpful in the recognition of some of these tumors. Table 17-1 shows the immunohistologic profile of specific adenocarcinomas based on their site of origin.86
Papillary Adenocarcinomas
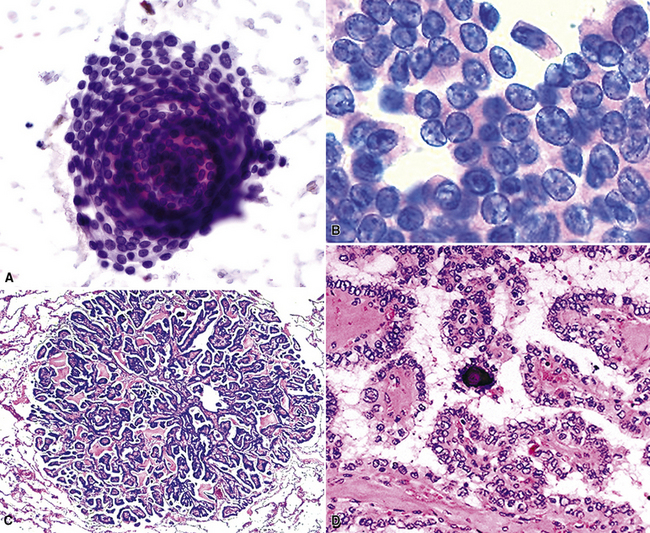
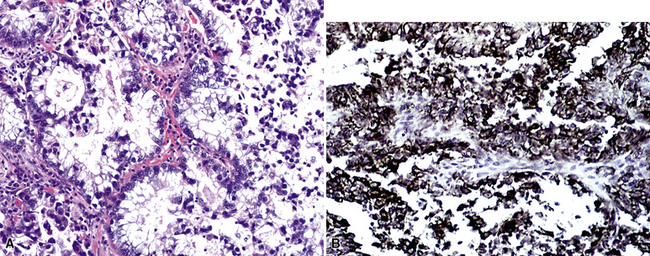
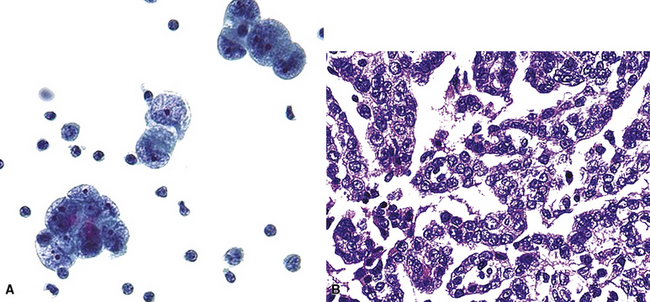
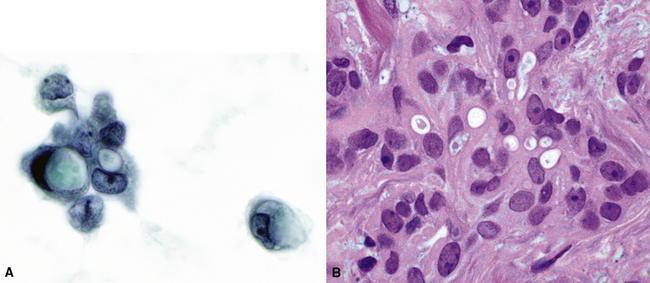
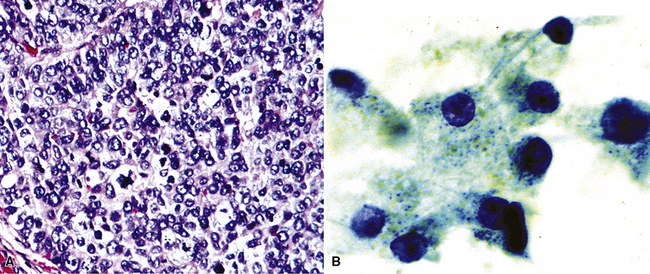
Silver and Askin reported that primary papillary pulmonary adenocarcinomas—defined as such if 75% or more of the neoplasm shows micropapillary architecture—are not uncommon87; moreover, micropapillae may be seen in conventional adenocarcinomas of the lung88,89 (Fig. 17-13). Metastatic adenocarcinomas in the lung also may contain micropapillary structures. In FNA specimens, such tumors show fibrovascular fragments covered by cuboidal or low-columnar neoplastic cells.
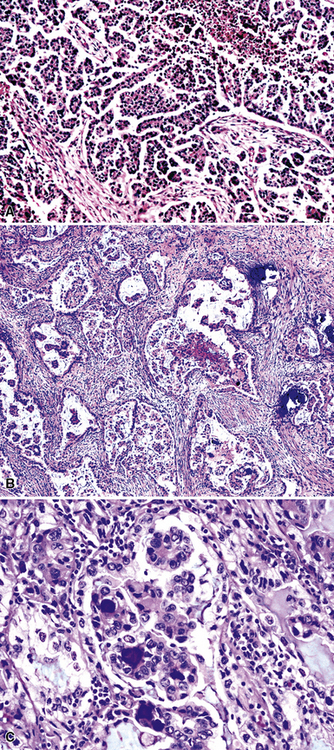
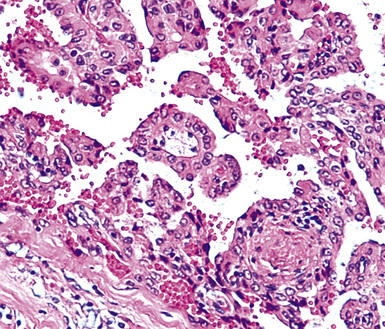
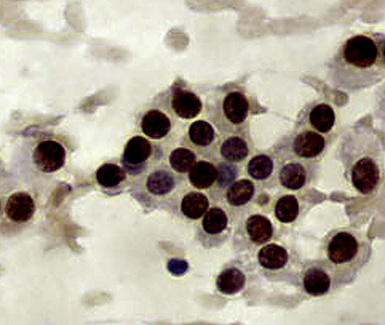
Metastatic papillary adenocarcinomas may originate in the thyroid, breast, ovary, or kidney (Fig. 17-14). Thyroid carcinomas of all histologic subtypes have the potential to metastasize to the lung. Of all thyroid carcinomas that spread to distant sites other than lymph nodes, up to 50% involve the pulmonary parenchyma.90–92 Papillary thyroid carcinoma almost always metastasizes to regional cervical lymph nodes beforehand90; in addition, this tumor type may directly invade the trachea and produce an endoluminal mass. Anaplastic thyroid carcinoma shares the latter potential. Hilar intrathoracic and mediastinal lymph nodes are also involved by papillary thyroid carcinoma in half of patients with lung metastasis.93 Secondary papillary thyroid carcinoma may grow very slowly and remain solitary for extended periods, simulating the biologic characteristics of a primary pulmonary neoplasm.90 In addition to its papillary substructure, other cytologic clues to metastatic papillary thyroid carcinoma include its characteristic nuclear features—including nuclear grooves, nuclear membrane irregularities, cytoplasmic invaginations (pseudoinclusions), and nuclear overlap—as well as the formation of colloid and psammoma bodies (Fig. 17-15).
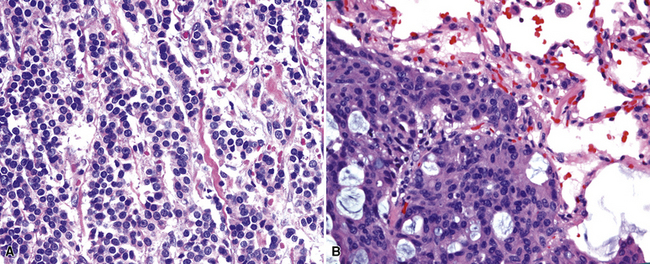
All types of ovarian carcinoma may metastasize to the lungs, and up to 50% of stage IV cases feature pulmonary involvement.94 The papillary serous form of ovarian cancer is the most common subtype. The pleura is often involved early, by lymphatic spread through the diaphragm, and the peripheral lung parenchyma is then affected. Malignant pleural effusions caused by papillary serous carcinomas are seen in 40% of all cases with metastases,94 and solitary pulmonary nodules are present in 7%.95 Lymphangitic intrapulmonary growth of ovarian malignancies is associated with a rapid demise.96,97
Immunopathologic studies to determine the site of origin of a papillary carcinoma in the lung are outlined in Table 17-2.86 The authors recommend using at least one marker (e.g., vimentin or pan-keratin) that should be positive in each of the differential diagnostic possibilities to establish the antigenic integrity of the tissue.98 In this setting, the highest specificity of immunopathologic identification is associated with tumors of thyroid, pulmonary, or mammary origin. Metastatic papillary tumors arising in the kidneys or ovaries are more difficult to distinguish from others definitively with immunohistology, although CA-125 reactivity is a consistent feature of ovarian epithelial neoplasms (Fig. 17-16).
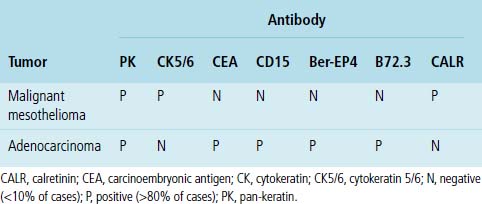
Another malignancy that often has a papillary “pseudocarcinomatous” appearance, especially in pleural fluid specimens, is the epithelioid variant of malignant mesothelioma (Fig. 17-17). Renshaw and colleagues estimated that the sensitivity of pleural fluid cytology for the diagnosis of mesothelioma was only 32%99 because epithelioid tumor cells often have a bland appearance and the sarcomatoid variant of mesothelioma rarely sheds into the pleural space. Many reports have considered the diagnostic distinction of mesothelioma from metastatic adenocarcinoma. This topic is discussed in detail in Chapter 20.100–102 Immunopathologic and electron microscopic studies are generally used to make this distinction.100,103 Table 17-3 shows a typical immunopathologic antibody panel that can be used to distinguish mesothelial proliferations from epithelial tumors.100,104,105 Imlay and Raab examined the utility of immunohistochemistry in this context in hospital practice.45 They reported that immunopathologic techniques were applied to 2.6% of all cases with pleural fluid specimens. In 71.9% of these cases, a firm interpretation was facilitated by the results of such analyses.45 However, none of the diagnoses in that series were based solely on immunopathology.45 The low prevalence of mesothelioma in the general population explains the rarity of this interpretation in the experience of most practicing pathologists.
Clear Cell Adenocarcinomas
Clear cell features are best seen in histologic specimens, in which the cytoplasm of the neoplastic cells is lucent and only the cell borders are apparent. Clear cell change is often an artifact of formalin fixation, and in cytologic specimens, the cytoplasm of the neoplastic cells has a more vacuolated appearance. Primary clear cell tumors of the lung are rare and have variable cellular lineage; they are usually peripherally located106 (see Chapters 16 and 19). Clear cell change also may be seen focally in common tumor types. For example, biopsy specimens of primary squamous cell carcinomas may show that alteration. That phenomenon is seen less frequently in cytologic specimens, in which the cytoplasm of the neoplastic cells maintains a classic “metaplastic” appearance.
Metastatic clear cell adenocarcinomas in the lungs may emanate from the kidney, breast, adrenal cortex, salivary gland, or other locations; primary clear cell malignancies have been described in practically every organ. The most common clear cell neoplasm in the lung is metastatic renal cell adenocarcinoma (Figs. 17-18 and 17-19).107 Hughes and associates reported that among 12 lung FNA specimens with clear cell features, 10 originated in the kidney, 1 in the cervix, and 1 in an undetermined site.108 Clear cell carcinomas are only a subset of renal tumors that may metastasize to the lungs; papillary, oncocytic, and sarcomatoid neoplasms may also do so. Because epithelial malignancies of the kidney have a proclivity to invade the renal veins and bypass the hepatic circulation, the first site of secondary disease may be in the lungs.107,109 The pulmonary parenchyma is involved in up to 75% of cases of metastatic renal cell carcinoma.109,110 Almost half of these patients have no symptoms that suggest extrathoracic disease110 (Fig. 17-20). Metastatic disease in the lungs may take several radiographic forms, including a solitary mass, multiple nodules, miliary spread (innumerable small nodules), large- or small-vessel emboli, lymphatic space disease, hilar or mediastinal lymph nodal disease, and endobronchial tumor.111–115 Metastatic renal cell carcinoma is an example of a neoplasm that may grow very slowly and become clinically evident only many years after the primary diagnosis.116
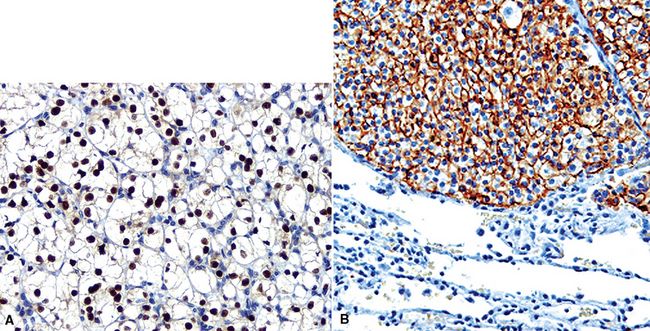
Like ovarian carcinomas, clear cell carcinomas of the kidney are difficult to identify definitively by immunohistologic studies. Most are reactive for CD10, PAX2, adipophilin, RCC antigen, and cytokeratin 8 (Figs. 17-21 and 17-22). In combination, these are highly suggestive of the diagnosis.117–120
Signet Ring Cell Adenocarcinomas
A “signet ring” cell is relatively small and has an eccentrically placed nucleus indented by a large cytoplasmic vacuole or multiple vacuoles. Such cells generally are considered part of the spectrum of poorly differentiated mucin-forming adenocarcinoma, and some tumors of that type are composed almost entirely of signet ring cell forms. Signet ring cell differentiation is uncommon in most primary pulmonary adenocarcinomas. If it is present, a secondary malignancy is favored (Fig. 17-23). Sources of metastases with that appearance include the stomach and other gastrointestinal sites, breast, and pancreas. Metastatic signet ring cell carcinomas are usually associated with a dismal prognosis; they characteristically spread initially to regional lymph nodes before involving the lungs. Some esophageal signet ring cell tumors originating in foci of Barrett esophagus may directly invade the lung or pleura. Metastatic pancreatic adenocarcinomas, including signet ring cell variants, often involve the liver and lungs.121 Multiple pulmonary nodules are virtually always seen rather than a solitary secondary lesion.121
Well-Differentiated Adenocarcinomas
The authors use the term well-differentiated adenocarcinoma in more than just a descriptive fashion to mean a malignant glandular proliferation that is morphologically difficult to differentiate from benign or reactive pulmonary proliferations; the nosologic term “minimal-deviation adenocarcinoma” also has been used in this context. The recognition of these tumors is often extremely difficult in cytologic specimens because of the lack of contextual architecture. Well-differentiated primary adenocarcinomas of the lung include some “conventional” (acinar) adenocarcinomas, selected bronchioloalveolar adenocarcinomas, and salivary gland-type adenocarcinomas (see Chapter 16). These show relatively low nuclear-to-cytoplasmic ratios and lack the degree of nuclear atypia seen in more overtly malignant lesions. Bronchioloalveolar adenocarcinomas, in particular, often pose a diagnostic conundrum, especially in cytologic preparations where the malignant cells lining the alveolar septa in a “lepidic” manner cannot be seen. However, well-differentiated adenocarcinomas in the lung may also be metastases, especially when sharply defined mass lesions are seen macroscopically. Sites of origin for secondary adenocarcinomas with these attributes include the breast, pancreas, kidney, thyroid, and salivary glands.
Mammary carcinomas may metastasize to the lungs, pleura, or both. In most cases, the malignant cells are easily identified, but in some FNA or pleural fluid specimens, the malignant cells are bland (Fig. 17-24). Intrapulmonary metastatic foci are often nodular, but other presentations, such as endobronchial lesions, lymphangitic spread, and intravascular tumor emboli, may be seen.122–125 Approximately 50% of metastatic breast cancers are associated with pleural effusions.33,126 Casey and colleagues reported that 3% of primary mammary carcinomas were associated with a lung mass at the time of initial diagnosis; 43% of the pulmonary lesions were metastases and 52% were concurrent primary lung cancers. The rest were non-neoplastic.125 The lungs and pleura are the first sites of tumor recurrence in 10% of cases of mammary carcinoma.124 In patients with a history of breast carcinoma and an adenocarcinoma in the lung, Raab and coworkers showed, with immunohistochemical studies (for estrogen receptor, gross cystic disease fluid protein-15, S-100 protein, and carcinoembryonic antigen) that 50% of the pulmonary lesions were metastatic mammary tumors, 37% were primary pulmonary carcinomas, and 13% were indeterminate (Figs. 17-25 and 17-26).127 Dabbs and associates found that some primary lung cancers may label for hormone receptor proteins, but not for the other specified markers.128 Mammaglobin is another breast-related polypeptide that is valuable in the immunohistochemical recognition of metastatic mammary carcinoma.129 On the other hand, positivity for thyroid transcription factor-1 (Fig. 17-27) is compelling evidence in favor of pulmonary derivation in this context, as discussed later.
Oncocytic and Granular Cell Carcinomas
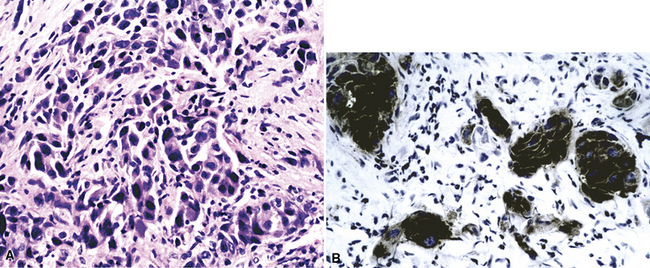
Neoplasms composed of cells containing granular cytoplasm may be oncocytic or nononcocytic. Both subtypes contain cells that have an eosinophilic appearance on conventional stains. In oncocytic cells, this reflects the presence of numerous cytoplasmic mitochondria. Nononcocytic cells instead contain a preponderance of other cytoplasmic organelles, especially lysosomes. Primary pulmonary malignancies that may have a granular cell constituency include conventional adenocarcinomas and salivary gland-type adenocarcinomas. However, this cytologic feature is rare in lung tumors. Secondary neoplasms with granular cytoplasm include carcinomas of the kidney, thyroid, and liver (Fig. 17-28).
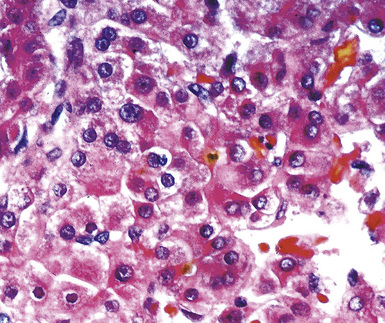
The lungs are involved in up to 70% of cases of metastatic hepatocellular carcinoma (HCC).130–132 Several patterns of intrapulmonary spread have been reported; through transdiaphragmatic lymphatics, HCC may enter the right lower lobe. In this setting, several parenchymal mass lesions and pleural involvement are typically seen.133,134 Alternatively, HCC may transit the venous system through the hepatic vein and inferior vena cava, presenting as a large intravascular mass or “showering” the lungs with small emboli that appear as miliary tumors.133 Cytologically, the cells of this neoplasm often show multinucleation; this feature is uncommon in most primary pulmonary malignancies. Moreover, bile formation may be seen in metastatic HCC (Fig. 17-29). In addition, the tumor cells cluster around intralesional blood vessels and “stripped” nuclei are seen.135 Cytopathologists must avoid misinterpretation of FNA specimens obtained from the right lower pulmonary lobe as well-differentiated oncocytic or granular cell carcinomas; these specimens may simply represent normal liver that has been mistakenly sampled instead of the lung. A monoclonal antibody raised against paraffin-embedded tissue from HCC, and designated “Hep-PAR1,” has shown reasonably good discrimination in labeling that tumor.136