Ventricular arrhythmias (VAs) are commonly reported after implantation of left ventricular assist devices (LVADs). Their relation to all-cause mortality and potential risk factors remains unclear. We conducted a meta-analysis of observational studies with the primary objective of evaluating the association of post-LVAD VAs with all-cause mortality at 60, 120, and 180 days. The secondary end point was the association of potential risk factors (cause of cardiomyopathy, indication for LVAD, and history of VA) with mortality in patients with post-LVAD VAs. We searched MEDLINE, Embase, and Cochrane Central from 2001 to 2015. Two reviewers independently searched, selected, and assessed quality of included studies with differences resolved by consensus. Data were collected and analyzed using random- and fixed-effect model, as appropriate, with inverse-variance weighting. Of 2,393 studies identified, 9 observational studies were eligible including 1,179 patients with a mean follow-up of 220 days. Post-LVAD VAs were associated with increased risk of all-cause mortality after adjusting for competing risk factors at 60 days (adjusted odds ratio [OR] 1.91, 95% confidence interval [CI] 1.18 to 3.11, p = 0.001), 120 days (adjusted OR 1.97, 95% CI 1.01 to 3.85, p = 0.05), and 180 days (adjusted OR 2.04, 95% CI 1.01 to 4.15, p = 0.05). Using meta-regression analysis, it was found that only history of VA was a risk factor for mortality after LVAD implantation. In conclusion, post-LVAD VA is associated with an increased risk of all-cause mortality with pre-LVAD VAs acting as a risk factor. This meta-analysis, despite being only hypothesis generating, sets the stage for prospective collection of VA information in a prospective device trial or in the Interagency Registry for Mechanically Assisted Circulatory Support.
Left ventricular assist devices (LVADs) have increasingly played a pivotal role in the management of medically refractory end-stage heart failure after the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial demonstrated survival benefit from LVADs in patients with end-stage heart failure. Recently, there has been a significant increase in the use of LVADs in the United States. These devices have been used as a bridge to recovery, bridge to transplant (BTT) , or as destination therapy (DT). Ventricular arrhythmias (VAs) are one of the most commonly reported adverse events in patients with LVADs, after bleeding and infectious complications. VAs have been observed to occur with a greater frequency after LVAD implantation, although reported incidence varies widely in reported observational studies and registries. Several clinical variables, including the presence of preexisting VAs, cause of cardiomyopathy, device type, indication for support, and duration of follow-up, have been reported to modulate the risk of post-LVAD VAs. However, the relative contribution of these potential risk factors remains unclear. There has been growing evidence that LVAD-induced unloading may actually reduce the risk of VA through reverse electrophysiological remodeling and reduction of QRS and QT intervals. Observational studies have reported an association between VAs and increased mortality in patients with LVADs, but the association is inconsistent. In view of the ambiguity surrounding the prognostic significance and management of VAs in the LVAD population, we conducted a systematic review and meta-analysis of observational studies with the primary objective of evaluating the association between post-LVAD VAs and all-cause mortality.
Methods
Our analysis is based on the guidelines of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. With the assistance of a trained research librarian, we searched MEDLINE, Embase, CINAHL, the Cochrane Controlled Trials Register, Cochrane Database of Systematic Reviews, and the National Institutes of Health ClinicalTrials.gov database from 2001 to 2015. In addition, we searched abstracts of reports published by the American College of Cardiology, the American Heart Association, the European Society of Cardiology, the European Heart Failure Society, and the Heart Failure Society of America. We chose the year 2001 as our initial time reference as the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial, published in 2001, was the first trial to demonstrate a survival benefit for LVAD in patients with advanced heart failure. We identified relevant studies using the following MeSH terms and keywords: “Tachycardia, Ventricular” OR “Ventricular Fibrillation” OR “Ventricular tachyarrhythmia” AND “Heart Failure” OR “Myocardial Failure” OR “Heart Decompensation” AND “Heart-Assist Devices” OR “Heart-Assist Pump” OR “Heart Assist Pump” OR “VAD” OR “LVAD” OR “Mechanical Circulatory Support” OR “Advanced Cardiac Therapy”. A manual search of references was also performed. Titles and abstracts were reviewed independently by 2 of the investigators (NM and CS). Differences were resolved by consensus and by a third reviewer (OM) when needed.
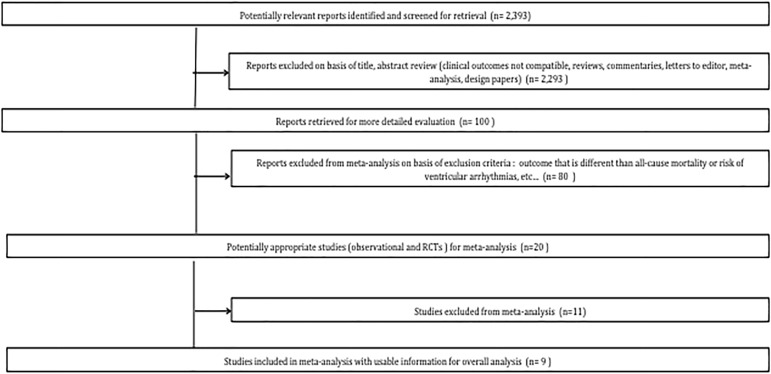
To our knowledge, no published, randomized controlled trials to date have evaluated the relation between post-LVAD VAs and all-cause mortality. We applied the following inclusion criteria in our review of potentially eligible studies: (1) observational studies (prospective and retrospective) exploring the association of VAs (after LVAD implantation) with all-cause mortality; (2) patients aged >18 years; and (3) studies of LVADs and not other types of VADs (right ventricular assist devices or biventricular assist devices). Studies were excluded if (1) outcome was different from all-cause mortality; (2) there was an inability to obtain both the numerator (i.e., number of patients experiencing a given outcome) and denominator for the intervention and control groups; (3) studies evaluated temporary assist devices (e.g., Impella, TandemHeart) or/and extracorporeal systems; and (4) studies had <30-day follow-up, as we are interested in long-term outcomes only. Figure 1 summarizes the results of the search. Studies were rated according to the Newcastle–Ottawa Scale used for assessing nonrandomized observational studies.
The primary end point of this meta-analysis was association of post-LVAD VAs implantation and all-cause mortality at 60, 120, and 180 days after implantation. These specific time points were chosen as a result of data availability and in an attempt to increase the yield of studies for the final analysis. The secondary end point was to delineate potential risk factors for all-cause mortality in patients with post-LVAD VAs.
Two independent reviewers (NM and CS) extracted the following data elements from each study: (1) publication details including first author’s last name and year; (2) study design; (3) characteristics of the study population which included number of patients with versus without ischemic cardiomyopathy, gender, mean age, number of patients on DT LVAD versus BTT LVAD, and number of patients on continuous flow LVAD (cf-LVAD) versus pulsatile flow LVAD (pf-LVAD); and (4) duration of follow-up. Differences were resolved by consensus and by a third reviewer (OM) when needed.
VAs were defined as any sustained ventricular tachycardia (VT) lasting for >30 seconds or ventricular fibrillation episode detected by implantable cardioverter defibrillator interrogation, telemetry, or electrocardiography or requiring defibrillation or antitachycardia pacing. Nonsustained VT was not included. DT was defined as patients who were not candidates for heart transplant and had LVAD implanted permanently. Pulsatile LVADs consisted of the first-generation devices implanted. These devices, such as the HeartMate XVE, were designed to mimic the physiologic action of the heart with an output defined by the displacement volume of the pumping chamber and pump rate. Disadvantages of these devices included inferior durability and large size with the attendant surgical complications. Cf- LVADs, such as the HeartMate II and Jarvik 2000, are newer generation devices that are smaller and are characterized by greater efficiency, less surgical trauma, and fewer complications compared with pulsatile devices.
Summary odds ratios (OR) and 95% confidence intervals (CI) were calculated for all clinical outcomes by pooling published results available for each study using standard meta-analytic methods. For all studies, multivariate regression analysis was performed to adjust for potential confounders (e.g., age, gender, inotropes, amiodarone, bleeding, thromboembolism, diabetes, and peripheral arterial disease). In addition, we performed an additional regression analysis to assess the effect of competing risk factors (right ventricular failure, pump thrombosis, stroke, and infection) after obtaining access to supplementary data from the studies’ investigators. However, we were not able to adjust for the Interagency Registry for Mechanically Assisted Circulatory Support score as only 2 studies reported it. Sensitivity analyses were performed to evaluate the robustness of the meta-analysis. Calculated ORs were transformed logarithmically. We assessed heterogeneity of the studies by calculating a Q statistic (significance defined as p <0.05), which we compared with the I 2 index ( I 2 ≥56% defined as significant). Data were collected and analyzed using a random- and fixed-effect model approach with inverse-variance weighting. The underlying heterogeneity further prompted us to perform metaregression analysis to investigate if our study end points (all-cause mortality) were affected by factors other than our primary treatment (i.e., LVAD). We adopted a weighted regression random-effect model and estimated between study variance (τ 2 ) using empirical Bayes estimate. A 2-sided p value <0.05 was regarded as significant for all analyses. All statistical calculations were performed using RevMan v5.0 software (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen). Potential publication bias was assessed with the Egger test and represented graphically with Begg funnel plots of the natural log of the OR versus its SE.
Results
The literature search yielded 2,393 potential studies. Of these, a total of 9 observational studies with a total population of 1,179 patients met our selection criteria ( Table 1 ). Studies were statistically heterogeneous with regard to all-cause mortality ( Q test, p <0.05 and I 2 = 78). Based on the Newcastle–Ottawa Scale, all included studies scored high in terms of selection, comparability, and outcome assessment ( Appendix Table 1 ). In patients who experienced post-LVAD VAs, most had VA early after implant (72%) and most of the documented VAs were sustained VT in nature ( Table 2 ).
| First Author | Year | N | FU (Days) | Cf-LVAD (%) | DT (%) | IC (%) | ICD (%) | Study design |
|---|---|---|---|---|---|---|---|---|
| Ambardekar | 2010 | 33 | 238 | 53 | 30.0 | 36.0 | 100% | Retrospective |
| Bedi | 2007 | 111 | 98 | 100 | 0.0 | 51.0 | NS | Prospective |
| Brenyo | 2012 | 61 | 662 | 100 | 28.0 | 61.0 | 0% | Retrospective |
| Cantillon | 2010 | 478 | 56 | 26 | NS | 59.0 | 19% | Retrospective |
| Enriquez | 2013 | 106 | 217 | 100 | 12.3 | 43.0 | 63% | Retrospective |
| Garan | 2013 | 94 | 276 | 100 | 51.0 | 52.1 | 82% | Prospective |
| Refaat | 2012 | 144 | 119 | 51 | 0.0 | 54.0 | 31% | Retrospective |
| Raasch | 2012 | 61 | 180 | 100 | 56.0 | 38.0 | 80% | Retrospective |
| Ziv | 2005 | 91 | 126 | 100 | <50% | 63.0 | 19% | Retrospective |
| First author | Patients with VAs | Patients with v-fib | Patients with sustained VT | Patients with early VA (< 30 days after implant) | Patients with late VA (> 30 days after implant) |
|---|---|---|---|---|---|
| Ambardekar | 8 (24%) | 1 (12%) | 7 (88%) | 5 (60%) | 3 (40%) |
| Bedi | 24 (22%) | 0 (0%) | 24 (100%) | 16 (67%) | 8 (33%) |
| Brenyo | 19 (31%) | 5 (26%) | 14 (74%) | 15 (79%) | 4 (21%) |
| Cantillon | 138 (29%) | 25 (18%) | 113 (82%) | 100 (72%) | 38 (28%) |
| Enriquez | 37 (35%) | 4 (12%) | 33 (88%) | 30 (81%) | 7 (19%) |
| Garan | 24 (23%) | 2 (8%) | 22 (92%) | 16 (67%) | 8 (33%) |
| Refaat | 29 (20%) | 4 (14%) | 25 (86%) | 23 (70%) | 6 (30%) |
| Raasch | 26 (43%) | 3 (13%) | 23 (87%) | 20 (77%) | 6 (23%) |
| Ziv | 32 (35%) | 7 (22%) | 25 (78%) | 25 (78%) | 7 (22%) |
| Total Patients | 347(29%) | 52 (15%) | 295 (85%) | 250 (72%) | 97 (28%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


