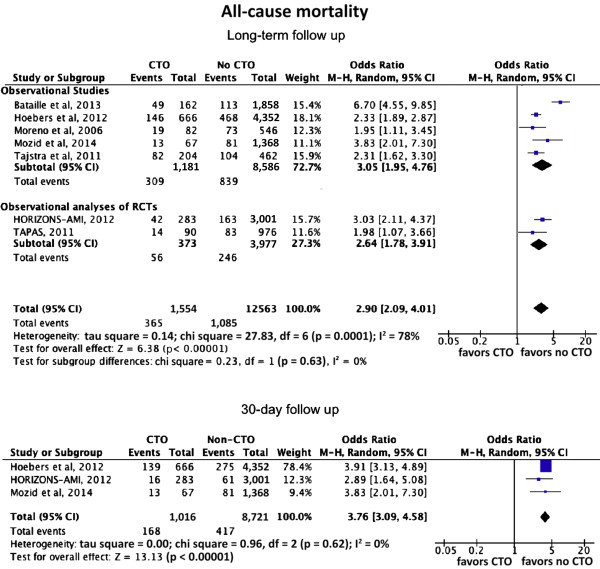
Several observational studies have compared clinical outcome in patients with a co-existing noninfarct-related artery chronic total occlusion (n-IRA CTO) versus those without, suggesting increased all-cause mortality. The goal of this study was to provide a systematic review and meta-analysis evaluating the impact of the presence of an n-IRA CTO on short- and long-term mortality after primary percutaneous coronary intervention. Studies published from January 1980 to January 2014 that compared the incidence of all-cause mortality in patients with ST-segment elevation myocardial infarction with co-existing n-IRA CTO versus those without were identified using an electronic search and reviewed using meta-analytical techniques. Seven studies (5 observational studies and 2 observational analyses of randomized controlled trials) comprising 14,117 patients and 1,554 patients (11.7%) with n-IRA CTO were included. The presence of n-IRA CTO was associated with increased incidence of all-cause mortality at a median follow-up of 25.2 months (interquartile range 24 to 60) compared with no CTO (absolute risk 23.5% vs 9.0%; odds ratio [OR] 2.90, 95% confidence interval [CI] 2.09 to 4.01; p <0.0001). This finding was consistent in the analysis of studies reporting 30-day follow-up (absolute risk 17.2% vs 4.7%; OR 3.79, 95% CI 3.13 to 4.59; p <0.0001). Co-existing n-IRA CTO was also associated with increased mortality in a subanalysis of patients with multivessel disease only (absolute risk 24.2% vs 11.3%; OR 2.23, 95% CI 1.90 to 2.63; p <0.0001). In conclusion, coronary CTO in the nonculprit artery in patients presenting with ST-segment elevation myocardial infarction is associated with increased short- and long-term all-cause mortality.
ST-segment elevation myocardial infarction (STEMI) is a dramatic cardiac event characterized by acute coronary thrombotic occlusion or subocclusion where rapid recanalization with primary percutaneous coronary intervention (PPCI) is the recommended optimal therapy. Multivessel disease (MVD) is present in a significant proportion of patients presenting with STEMI, and it has been widely reported that these patients have a worse prognosis than those with single-vessel disease (SVD) in the setting of acute myocardial infarction (MI), especially when concurrent cardiogenic shock (CS) is present. Several recent studies have suggested that the presence of a concurrent noninfarct-related artery chronic total occlusion (n-IRA CTO) in patients with MVD is the key factor driving the poorer survival seen in this patient group. However, not all data have confirmed n-IRA CTO as an independent predictor of mortality. In this respect, we performed a meta-analysis of the data examining the clinical impact of the presence of n-IRA CTO in patients presenting with STEMI treated with PPCI.
Methods
Our meta-analysis adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements for reporting on systematic reviews. We conducted the meta-analysis in accordance with the general guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2.
We conducted Cochrane Controlled Trials Registry and MEDLINE and EMBASE database searches for published articles from January 1980 to January 2014 using the following predefined search terms, “chronic total occlusion” OR “chronic occlusion” AND “myocardial infarction” OR “ST elevation myocardial infarction” OR “primary PCI.” Abstracts from selected major cardiology scientific meetings (American Heart Association, American College of Cardiology, European Society of Cardiology, and Transcatheter Cardiovascular Therapeutics) were reviewed. References from reviews and selected articles were also reviewed for potential relevant citations. We used no language restrictions. Studies were selected by 2 independent reviewers (SAO and FS).
We restricted our analysis to trials that met all the following inclusion criteria: (1) included patients presenting with STEMI, treated with PPCI; (2) either single-group cohort or a controlled comparison between patients with and without CTO; and (3) available data on long-term (at least 1 year) all-cause mortality ± short (either 30 days or inhospital). Randomized controlled trials, registries, and prespecified subgroups of studies reporting data on CTO in STEMI were considered for analysis. Full-text articles and meeting abstracts were included.
Exclusion criteria were duplicate reports and ongoing studies. Data extraction and information on study design and clinical and safety outcomes were performed independently by 2 reviewers (SAO and FS). Discrepancies were resolved by consensus.
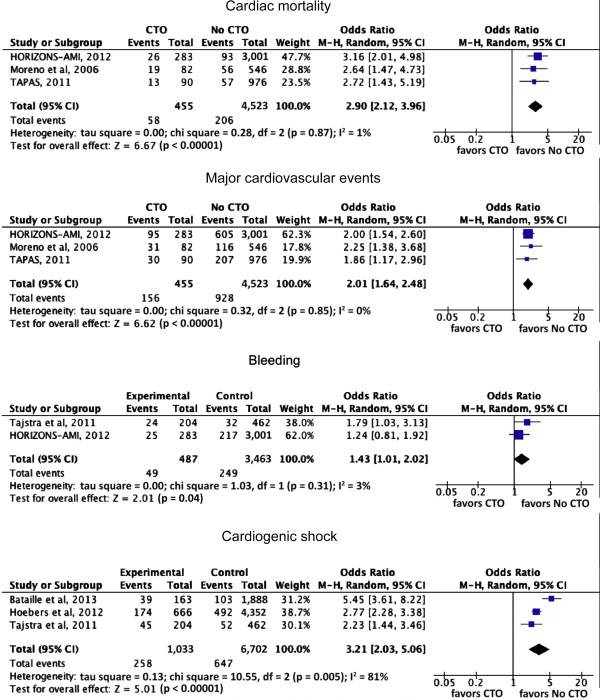
The primary efficacy end point was mortality (by any cause). Secondary end points included cardiac mortality and the composite end point of major cardiac events and the individual components of MI, as defined in each study. Major bleeding and CS were also assessed. Outcomes were based on the longest follow-up available for each study. The same analyses were performed on short-term outcome defined as either inhospital or 30 days. The quality of nonrandomized studies included in the meta-analysis was assessed for descriptive purposes by the Newcastle-Ottawa Scale for cohort studies ( http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm )
To provide an estimation on the association between the presence of CTO and clinical outcomes, a global analysis was performed comparing outcome in patients with n-IRA CTO versus those without CTO that included patients with SVD and MVD. To examine the potential confounding influence of MVD, a subanalysis was performed comparing outcome in n-IRA CTO versus patients with MVD and without CTO. The results of studies were combined using a random model because we anticipated potential heterogeneity across the studies, especially between the observational studies. The results were confirmed by the Mantel-Haenszel fixed-effect model to avoid small studies being overly weighted. The Cochrane Q statistic and the I 2 ; statistic were used to assess the heterogeneity across trials. A Cochrane Q statistic with a p value ≤0.1 will be considered significant. The I 2 statistic was used to measure the consistency among trials with values of 25%, 50%, and 75% showing, respectively, low, moderate, and high heterogeneity. A funnel plot was used to assess for the presence of publication and other reporting biases by plotting the standard error against the log risk ratio. Using Egger’s regression method, we examined the association between the study size and estimated treatment. Analyses were conducted by RevMan software, version 5.0 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen).
Results
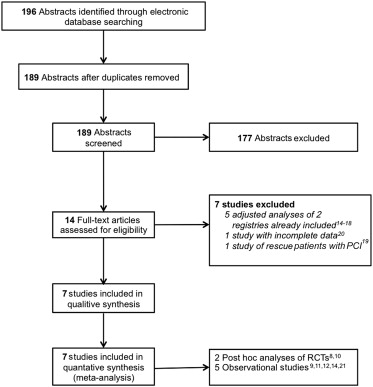
Our search identified 189 reports, of which 177 were excluded based on abstract review ( Figure 1 ). Fourteen full-text articles were assessed for eligibility. Of these, 5 studies were excluded as they were previously performed adjusted analyses of 2 registries that met our inclusion criteria, including 3 analyses on the influence of gender, diabetes, and LV function, respectively, and 2 analyses on the influence of CS on mortality in the setting of n-IRA CTO. One observation study was excluded as the patients with STEMI were treated by rescue and not PPCI, and 1 observational study was excluded as patients presenting with NSTEMI were included with no specific data available on the patients presenting with STEMI. Finally, 7 studies representing a total of 14,117 patients were included in the analysis; of those, 2 were observational analyses of RCTs and 5 were observational studies. Details of the design of the included studies are listed in Table 1 . Three studies compared outcomes among 3 patients groups: SVD, MVD without CTO, and MVD with CTO, apart from 1 study where patients with SVD only were excluded from the analysis. The quality of the studies was assessed using the Newcastle-Ottowa scale ( Table 2 ).
| Source | Design | No. of patients | Period of enrollment | Female n(%) | Diabetes n(%) | Prior MI n(%) | Prior PCI n(%) | Cardiogenic Shock n(%) | % SVD | % MVD -n-IRA CTO | % MVD +n-IRA CTO | Clinical Endpoints | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mozid et al, 2014 | Retrospective cohort study | 1435 | Sep 2009-Nov 2011 | 395(27.5) | 158(11) | 155(10.8) | 91(6.3) | 111(7.7) | 95.3 | 4.7 | Death, LVEF, TIMI 2/3 flow | 30 days, 2.1 years mean | |
| Bataille et al, 2013 | Retrospective cohort study | 2020 | 2006-2011 | 480(23.7) | 246(12.1) | 238(11.7) | 188(9.3) | 246(12.1) | 70 | 22 | 8 | Death | 1 year |
| Claessen et al, 2012 (HORIZONS-AMI) | Subgroup analysis of RCT | 3283 | NA | 754(22.9) | 535(16.3) | 348(10.6) | 350(10.6) | NA | 46.4 | 45 | 8.6 | MACE, Death, CV Death, ReMI, Stroke, Major Bleeding, IDTVR, ST, TIMI 3 flow, | 30-day, 30-day to 3-year, overall 3-year |
| Hoebers et al, 2012 | Retrospective cohort study | 5018 | Jan 1997-2008 | 1411(28.1) | 563(11.1) | 686(13.7) | NA | 609(12.1) | 64 | 23 | 13 | Death, LV function | 30 days, 5 year |
| Tajstra et al, 2012 | Retrospective cohort study | 666 | Jan 1999 to Dec 2004 | 187(28.1) | 163(25) | 202(30) | NA | 97(15.0) | 0 | 69 | 31 | Death, LVEF, Max CK, Hospital stay, Re-occlusion, CABG during hosp, CABG at discharge, GI bleeding. | In-hospital, 5-year |
| Lexis et al, 2011 (TAPAS) | Subgroup analysis of RCT | 1071 | Jan 2005- Dec 2006 | 316(29.5) | 123(11.5) | 101(9.9) | NA | NA | 91.6 | 8.4 | Death, blush grade, St segment resolution | 1 year | |
| Moreno et al, 2006 | Retrospective cohort study | 630 | Before 2003 | 203(32) | 218(34.6) | 133(21) | 73(11.5) | NA | 54.8 | 31.9 | 13.3 | Death, Cardiac Death, ReMI, TVR, MACE | 30-day and 2-year |
| Name | Design | Selection | Comparibility | Outcome | Total |
|---|---|---|---|---|---|
| Mozid et al, 2014 | Cohort | **** | ** | ** | 8 |
| Bataille et al, 2013 | Cohort | **** | ** | ** | 8 |
| Claessen et al, 2012 (HORIZONS-AMI) | Subgroup analysis of RCT | **** | ** | *** | 9 |
| Hoebers et al, 2012 | Cohort | **** | * | *** | 8 |
| Tajstra et al, 2012 | Cohort | **** | * | *** | 8 |
| Lexis et al, 2011 (TAPAS) | Subgroup analysis of RCT | **** | ** | *** | 9 |
| Moreno et al, 2006 | Cohort | *** | ** | * | 6 |
In the global analysis of all studies, the presence of an n-IRA CTO was associated with increased mortality at a median long-term follow-up of 30 months (interquartile range 12 to 60) compared with non-CTO (including SVD or MVD without CTO; absolute risk 23.7% vs 9.0%; odds ratio [OR] 2.79, 95% confidence interval [CI] 1.96 to 3.99; p <0.0001). The results were consistent across the observational analyses of RCTs and the observational studies analyses ( Figure 2 ). Short-term all-cause mortality (inhospital or 30 days) was reported in 3 studies (n = 8,968 patients). There was a significant association between CTO and increased short-term mortality (absolute risk 17.2% vs 4.7%; OR 3.79, 95% CI 3.13 to 4.59; p <0.0001; Figure 3 ).