Drug-eluting stent (DES) in-stent restenosis (ISR) can be treated by restenting using the same DES as previously placed (same stent strategy), versus switching to a stent that elutes a different drug (different stent strategy). To compare the efficacy of these strategies, a meta-analysis of controlled trials and observational studies evaluating patients with DES ISR was performed. The primary outcome was target lesion revascularization or target vessel revascularization, and secondary outcomes were major adverse cardiovascular events, death, and myocardial infarction. Pooled odds ratios (ORs) were calculated with the generic inverse variance method using a random-effects model. The chi-square test was used to evaluate heterogeneity. Ten studies (1,680 patients) were included. There was no significant heterogeneity among the studies for any end point. The different stent strategy was found to reduce the odds of target lesion revascularization or target vessel revascularization (OR 0.73, 95% confidence interval [CI] 0.55 to 0.96) and major adverse cardiovascular events (OR 0.72, 95% CI 0.54 to 0.96). There was no difference between the 2 strategies in rates of death (OR 1.03, 95% CI 0.49 to 2.16) or myocardial infarction (OR 0.59, 95% CI 0.24 to 1.41). In conclusion, this study demonstrates that treatment of DES ISR by restenting with a different DES than previously placed may lead to improved outcomes compared with the use of the same DES. Further large-scale trials are needed to confirm this effect.
The optimal method of dealing with drug-eluting stent (DES) in-stent restenosis (ISR) is unclear. Competing strategies include restenting using the same DES as previously placed, versus switching to a stent that elutes a different drug (the so-called switch strategy). Although there have been a multitude of observational studies and 1 randomized clinical trial evaluating this, the method that would lead to the best possible patient outcome remains uncertain. This is partly a function of small sample sizes and limited statistical power of these individual studies. In the current literature, there is no existing meta-analysis that addresses this question. The aim of this meta-analysis was to evaluate the sum of available evidence in this regard and decipher if either strategy is superior over the other.
Methods
This meta-analysis was performed in accordance with the MOOSE and PRISMA checklists. PubMed, Scopus (including EMBASE), and Web of Knowledge databases were searched using the following search terms: “drug-eluting stent restenosis,” “switch strategy,” “same stent,” “different stent,” “homo-stent,” and “hetero-stent.” The search was limited to articles in English and spanned the entire duration of these databases from inception to February 2013. A manual search was also performed using references from selected articles and the “related results” section of PubMed. Only articles published in peer-reviewed journals were considered for inclusion.
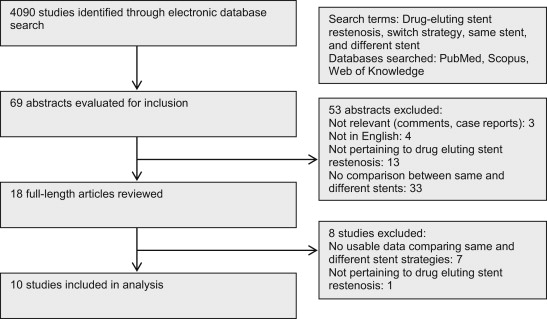
To be considered for inclusion, studies needed to (1) evaluate patients with DES ISR (with at least the large majority of patients having de novo DES ISR) as the study population, (2) contain a comparison of same stent (SS) and different stent (DS) strategies, even if only as a subgroup, and (3) evaluate at least one of the following outcomes: target lesion revascularization (TLR) or target vessel revascularization (TVR), major adverse cardiovascular events (MACEs), death, or myocardial infarction (MI). Exclusion criteria were (1) the absence of a direct comparison group and (2) the absence of extractable data, in the form of odds ratios (ORs), hazard ratios, or absolute event rates ( Figure 1 ).
The rate of TLR or TVR was used as the primary end point. Secondary end points included MACE, death, and MI. For MACE, definitions varied among individual studies; these were noted and included in the table for study characteristics ( Table 1 ), but the event rates and measures of effect size were taken as such.
| First Author (Study Name) | Year | Study Design | Centers | Mean Age (yrs) | Women (%) | Total Patients | Initial Stent | Intervention Stent | MACE Definition | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Cosgrave | 2007 | Cohort | 2 | 63 | 10 | 174 | SES 62.2%, PES 37.8% | SS: SES 72%, PES 28%; DS: SES 48.9%, PES 51.1% | Death, MI, TVR | 26 months |
| Garg | 2007 | Cohort | 1 | 64 | 36 | 116 | NA | NA | Death, MI, TVR | 12 months |
| Mishkel | 2007 | Cohort | 1 | 66 | 27 | 77 | SES 93%, PES 7% | SS: SES 95.3%, PES 4.7%; DS: SES 13.6%, PES 86.4% | Death, MI, TLR | NA |
| Liistro | 2009 | Cohort | 2 | 66 | 18 | 68 | NA | SS: SES 86%, PES 14%; DS: SES 61.3%, PES 38.7% | Death, MI, TLR, ST | 16 months |
| Sardella | 2009 | Cohort | 1 | 62 | 26 | 46 | SES, PES, TES (no split available) | NA | Death, MI, TVR | 25 months |
| Ge | 2010 | Cohort | 2 | 66 | 29 | 41 | NA | SES 73.9%, PES 23.9%, TES 2.2% | Death, MI, TVR | 20 months |
| Mehilli (ISAR-DESIRE 2) | 2010 | RCT | 2 | 67 | 23 | 450 | SES 100% | SES 50%, PES 50% | Death, MI, TLR | 12 months |
| Alfonso (RIBS III) | 2012 | Cohort | 12 | 66 | 27 | 325 | SES 26%, PES 44%, ZES 12%, EES 12%, other 5% | SES 36%, PES 31%, ZES 2%, EES 31% | Cardiac death, MI, TLR | 771 days |
| Ko | 2012 | Cohort | 20 | 62 | 34 | 268 | SES 45.7%, PES 40.5%, ZES 13.8% | SES 61.2%, PES 26%, ZES 6.9%, EES 4.5% | Death, MI, TVR | 973 days |
| Freixa | 2013 | Cohort | 1 | 64 | 35 | 115 | SES 61%, PES 33.6%, ZES 3.1%, EES 2.3% | SS: SES 90.2%, PES 9.8%; DS: SES 37.3%, PES 34.1%, ZES 7.6%, EES 20.8% | NA | 22 months |
Two investigators (AV and AM) evaluated each study for inclusion and extracted data in duplicate using a standardized protocol and reporting form. These variables included year of publication, type of study, number of centers, age and gender composition, patients in each group and total number of patients, study definition of MACE, follow-up duration, and outcome variables ( Table 1 ). Differences were resolved by consensus. Risk of bias in the included studies was evaluated using the Downs and Black risk assessment tool.
Data were extracted on an intent-to-treat basis, using the number of patients as the denominator (compared with the number of lesions). Adjusted ORs were used for analysis when available. Funnel plots were created to visually assess publication bias. Heterogeneity of the studies was analyzed using the Cochrane Q statistic chi-square test, for which a p value <0.2 signified potential heterogeneity. An I 2 test, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance, was also performed for each of the comparisons. Heterogeneity was considered low, moderate, substantial, and considerable, based on I 2 values of 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100%, respectively. Finally, results of a prespecified subgroup analysis comparing results in single- and multicenter studies were also used for this purpose.
The pooled OR was used as the measure of effect in the overall comparison test, and study data were combined using the generic inverse variance pooling method. A random-effects model was used for all analyses. Results for individual trials and summary results are expressed as ORs with 95% confidence intervals (CIs). A 2-sided p value <0.05 was considered to be statistically significant.
All statistical calculations were performed using Review Manager (RevMan [Computer program], version 5.1; The Nordic Cochrane Centre, The Cochrane Collaboration, 2011, Copenhagen, Denmark).
Results
The systematic review of the literature yielded 4,090 studies, of which 18 full-length articles were evaluated for inclusion ( Figure 1 ). Eight studies were excluded: 7 as they did not have usable data comparing outcomes between SS and DS strategies and 1 as it did not pertain to the DES ISR population ( Supplementary Table 1 ). Ten published studies, containing 1,680 patients, were used for the final analysis ( Table 1 ). All except one (the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) trial, a randomized controlled trial) were cohort studies, and follow-up ranged from 12 to 32 months. Using the Downs and Black risk assessment tool, most articles were found to have an intermediate risk of bias (median score 23, range 22 to 29; Supplementary Table 2 ).
The studies were found to be homogenous, with an I 2 of 0% for all outcomes. Although a review of funnel plots for TLR or TVR and MACE did not show obvious publication bias, this risk cannot be eliminated because of the small sample size and lack of small studies showing benefit with an SS strategy ( Supplementary Figures 1 and 2 ).
Nine studies, comprising 1,639 patients, were used for analysis of the primary end point, TLR or TVR. The event rate was 19.5% (102 of 523) in the DS arm compared with 23.9% (129 of 540) in the SS arm (2 studies did not have raw event rate data for the 2 arms and had only adjusted ORs). The DS strategy was found to decrease the odds of TLR or TVR by almost 30% compared with the SS strategy, and this difference was statistically significant (pooled OR 0.73, 95% CI 0.55 to 0.96, p = 0.02; Figure 2 ).
When MACE was evaluated as the outcome of interest (8 studies), there was again a significant reduction in event rates in the patients treated with a DS strategy compared with those treated with an SS strategy (18.1%, 81 of 448 vs 24.2%, 127 of 524; pooled OR 0.72, 95% CI 0.54 to 0.96, p = 0.03; Figure 3 ). No significant difference was found when comparing the 2 groups with regards to death (7 studies; 3.1%, 16 of 523 vs 3.5%, 19 of 540; pooled OR 1.03, 95% CI 0.49 to 2.16, p = 0.95) or MI (7 studies; 1.3%, 7 of 523 vs 2.8%, 15 of 540; pooled OR 0.59, 95% CI 0.24 to 1.41, p = 0.23; Figures 4 and 5 ).




