Optimal management of perioperative anticoagulation in patients undergoing pacemaker or implantable cardioverter–defibrillator implantation is not yet established. We performed a meta-analysis of the published literature to assess the safety and efficacy of perioperative heparin-based bridging therapy versus uninterrupted warfarin therapy in patients undergoing pacemaker or implantable cardioverter–defibrillator implantation. We performed a systematic review of MEDLINE (1950 to 2012), EMBASE (1988 to 2012), Cochrane Controlled Trials Register (fourth quarter 2011), and reports presented at scientific meetings (1994 to 2011). Randomized controlled trials, case–control, or cohort studies comparing the safety and efficacy of uninterrupted warfarin therapy to heparin-based bridging therapy were eligible. Outcomes reported in eligible studies were rates of bleeding and thromboembolic events. Of 3,195 reports initially reviewed, we identified 8 studies enrolling 2,321 patients for the meta-analysis. Maintenance of therapeutic warfarin was associated with significantly lower bleeding postoperatively compared to heparin-based bridging therapy (odds ratio 0.30, 95% confidence interval 0.18 to 0.50, p <0.01). There was no significant difference in risk of thromboembolic events between these 2 strategies (odds ratio 0.65, 95% confidence interval 0.14 to 3.02, p = 0.58). In conclusion, strategy of uninterrupted warfarin therapy throughout pacemaker or implantable cardioverter–defibrillator implantation is associated with decreased risk of bleeding without increasing risk of thromboembolic events. This strategy is a viable alternative to heparin-based bridging therapy.
Many patients taking warfarin to prevent thromboembolism receive pacemakers and implantable defibrillators, collectively known as cardiovascular implantable electronic devices (CIEDs). Perioperative management of anticoagulation is a challenging clinical problem that requires balancing the risk of acute thrombosis against perioperative bleeding. Although current guidelines recommend suspending warfarin and using heparin-based “bridging” therapies, recent trials have suggested that there is no associated risk of perioperative bleeding by maintaining therapeutic warfarin throughout the implantation procedure. This study used a meta-analysis to systematically compare the safety and efficacy of uninterrupted warfarin therapy to heparin-based bridging therapy during CIED implantation.
Methods
We searched MEDLINE (1950 to 2012), EMBASE (1988 to 2012, week 24), Cochrane Controlled Trials Register (fourth quarter 2011), and reports presented at scientific meetings (1994 to 2011) published in English. We performed searches using a Web-based search engine (Ovid) with an explode option for each subject term and the option “and” for combining keywords. MEDLINE was searched from January of 1950 to week 4 of February 2012 using the terms “anticoagulants,” “implantable defibrillator,” “cardiac resynchronization therapy devices,” “heparin,” “low-molecular-weight heparin,” “pacemaker,” and “artificial” with a previously developed MEDLINE search strategy to retrieve the strongest studies of treatment. The EMBASE database and Cochrane Controlled Trials Register were searched from January 1988 to week 8 of 2012 with keywords “defibrillator,” “artificial heart pacemaker,” and “anticoagulant agent.”
To identify studies reported at scientific meetings, we searched the annual scientific sessions of the American College of Cardiology (1994 to 2011), American Heart Association (1994 to 2011), European Society of Cardiology (1994 to 2011), and North American Society of Pacing and Electrophysiology/Heart Rhythm Society (1994 to 2011). We conducted additional searches using the names of 11 authors frequently cited in narrative reviews on this subject, modified versions of the Cochrane Optimal Search Strategy, and bibliographies of the 10 most recent narrative review articles.
Eligible studies were randomized controlled trials, case–control studies, or cohort studies comparing the safety and efficacy of uninterrupted warfarin therapy to heparin-based bridging. Outcomes reported in these studies were bleeding and thromboembolic events. “Randomized controlled trial” was defined according to the Consolidated Standards of Reporting Trials statement and “case–control and cohort studies” were defined according to the Strengthening the Reporting of Observational Studies in Epidemiology statement. We only included full reports of articles published in peer-reviewed journals to minimize bias and allow full evaluation of studies.
Two independent reviewers (H.G. and H.A.) evaluated the studies for inclusion into the meta-analysis. A third blinded reviewer (F.P.) resolved any disagreements between reviewers. Reviewers were blinded to the authors, journals, and study source institutions. Authors were contacted to obtain additional data not reported in the original reports.
Methodologic quality was assessed using the Methodological Index for Non-Randomized Studies (MINORS). Quality assessment was conducted independently and in duplicate by 2 investigators for each article (H.G. and W.S.P.). Studies were scored on 12 items: aim of study, inclusion of consecutive patients and participation rate, prospective data collection, end points appropriate to the aim of the study, unbiased assessment of study end points, appropriateness of follow-up time after diagnosis, inclusion of loss to follow-up, prospective calculation of study size, comparable control group, contemporary control groups, baseline equivalence of groups on several factors, and adequate statistical analysis. Studies received 0 point to 2 points for each of these 12 components. Total score ranged from 0 point to 24 points. Low-quality and high-quality studies were defined as earning <16 and ≥16 points, respectively, on the MINORS test.
The principal measurement of effect was odds ratio (OR). ORs from each included trial were pooled using fixed- and random-effects models that used weighting based on inverse variance calculated according to DerSimonian and Laird. The Q test and I 2 index were used to check for quantitative heterogeneity of results with a p value <0.05 deemed statistically significant. We assigned low heterogeneity with I 2 <25%, moderate heterogeneity with I 2 ≥25% to 50%, and high heterogeneity with I 2 ≥50% to 75%. Where significant and moderate to high statistical heterogeneity was identified, a random-effect estimate was used preferentially as the summary measurement. We did not include studies without any events in the final analysis. Standardized mean difference was used for analysis of mean length of stay. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual trials and recalculating pooled OR estimates for the remaining studies. We also performed sensitivity analysis to investigate the effect of choice of summary statistic by repeating the analysis for OR, risk ratio, and risk difference. This was performed to ensure results were similar for all summary statistics and did not affect the overall conclusions of our study.
Publication bias was assessed using a funnel plot and an adjusted rank-correlation test according to the method of Begg and Mazumdar. All statistical analyses were done with Comprehensive Meta-Analysis 2.0 (Englewood, New Jersey).
Range of bleeding event definitions included palpable hematoma >2 cm anterior to the pulse generator, extracardiac bleeding or pocket hematomas that required intervention, discontinuation of anticoagulation therapy, or prolonged hospitalization ( Table 1 ). In 3 randomized trials, criteria for high-risk patients for thromboembolism were any of the following: (1) mechanical prosthetic valves; (2) atrial fibrillation with previous stroke, transient ischemic attack, systemic embolic event, mitral stenosis, or prosthetic heart valve; (3) atrial fibrillation with at least criteria of intermediate risk of embolic events: hypertension, diabetes, and left ventricular ejection fraction <35% or age >75 years; (4) intracavitary thrombi; and (5) recent deep vein thrombosis. There were differences in outcome definitions, anticoagulant regiments, and patient populations. There were more qualitative differences among the 4 cohort studies. One cohort study did not report exact criteria for patients with high risk of thromboembolism. The other 4 studies defined patients as high risk for thromboembolism if they had (1) atrial fibrillation with high risk of thromboembolic events, (2) mechanical heart valves, (3) venous thromboembolism, (4) intracavitary thrombus, and (4) cerebral vascular accident/transient ischemic attack. We also summarized the key differences in outcome definitions, anticoagulant regiments, and patient population.
| Design | Subjects | Uninterrupted Warfarin | Heparin-Based Bridging | Follow-Up (weeks) | Type of Heparin Used Postoperatively | Timing of Heparin Administration (hours) | |
|---|---|---|---|---|---|---|---|
| Ahmed et al | Retrospective cohort | 459 | 27% | 48% | 8 | UFH or LMWH | UFH 12, LMWH 24 |
| Ghanbari et al | Retrospective cohort | 123 | 23% | 16% | 4 | UFH | 6 |
| Tischenko et al | Prospective cohort | 272 | 14% | 43% | 1 | LMWH | 24 |
| Tolosana et al | Prospective RCT | 101 | 50% | 50% | 6 | UFH | 24 |
| Milic et al | Prospective RCT | 81 | 51% | 49% | 8 | UFH | 8 |
| Cheng et al | Prospective RCT | 100 | 50% | 7% | 6 | UFH or LMWH | 24 ⁎ |
| Li et al | Retrospective cohort | 766 | 42% | 26% | 4 | UFH or LMWH | 24 ⁎ |
| Cano et al | Retrospective cohort | 419 | 31% | 50% | 1 | LMWH | 12–24 |
⁎ Heparin type, timing of administration, and number of patients who received each heparin type were obtained by contacting the authors.
There were notable differences in procedures performed in the included studies. Procedures performed included generator changes, permanent pacemaker, implantable cardioverter–defibrillator, and cardiac resynchronization devices. Operator experience was variable among studies and some trials did not report the specific type of procedures performed.
Results
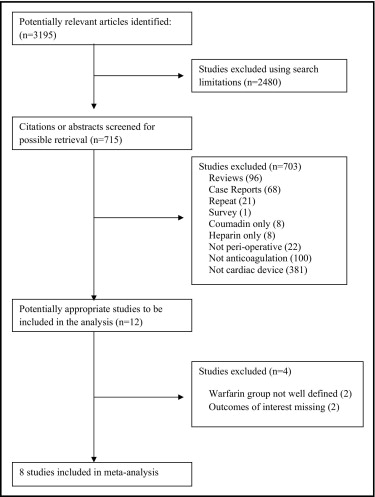
Of 3,195 studies evaluated, we identified 8 studies (5 cohort studies, 3 randomized trials) enrolling2321patients for the meta-analysis ( Figure 1 ). Baseline characteristics of the patients enrolled in these trials, indications for warfarin therapy, preoperative international normalized ratio, procedure type, bleeding definition, and concomitant use of antithrombotic agents are presented in Tables 1 and 2 . Table 3 presents the quality of these trials based on MINORS criteria. Q 2 test score was 12.19 and I 2 index was 42.58 indicating moderate heterogeneity (p = 0.09). There was no evidence of significant publication bias according to the rank correlation test of Begg and Mazumdar (tau = −0.32, p = 0.26).
| Indication for Warfarin | Preoperative INR | Antithrombotic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AF | Valvular Prosthesis | DVT/PE | TIA/CVA | Uninterrupted Warfarin | Heparin-Based Bridging | Uninterrupted Warfarin | Heparin-Based Bridging | |||
| ASA | C | ASA | C | |||||||
| Ahmed et al | — | 9% | 66% | 0% | 2.6 ± 0.5 | 1.3 ± 0.2 | + (57%) | + (13%) | + (77%) | + (13%) |
| Ghanbari et al | 36% | 4% | 0% | 0% | 2.4 ± 0.3 | 1.35 ± 0.3 | — | — | — | — |
| Tischenko et al | 42% | 10% | 22% | 11% | 2.2 ± 0.4 | 1.2 ± 0.2 | + (34%) | 0 | + (35%) | 0 |
| Tolosana et al | 78% | 59% | 22% | 40% | 2.0 ± 0.3 | 1.1 ± 0.2 | — | — | — | — |
| Milic et al | 89% | 6% | 55% | 0% | — | — | + (100%) | 0 | + (100%) | 0 |
| Li et al | 73% | 17% | 55% | 3% | 2.0 ± 0.5 | 1.5 ± 0.4 | + (53%) | + (5%) | + (39%) | 2 (2%) |
| Cheng et al | 65% | 18% | 0% | 7% | 2.2 ± 0.8 | 1.3 ± 0.3 | + (100%) (ASA or C) | + (100%) (ASA or C) | + (72%) (ASA or C) | + (72%) (ASA or C) |
| Cano et al | 72% | 22% | — | 11% | 2.5 ± 0.6 | 1.3 ± 0.2 | — | — | — | — |
| Source | Aim ⁎ | Rate † | Data ‡ | Measurement § | Bias ∥ | Time ¶ | Loss # | Size ⁎⁎ | Control †† | Contemporary ‡‡ | Factor §§ | Analysis ∥∥ | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al | 2 | 2 | 0 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 17 |
| Ghanbari et al | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 16 |
| Tischenko et al | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 19 |
| Tolosana et al | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 23 |
| Milic e tal | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 18 |
| Li et al | 2 | 2 | 0 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 17 |
| Cheng et al | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Cano et al | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 21 |
† Inclusion of consecutive patients and participation rate.
‡ Prospective data collection.
§ End points appropriate to aim of study.
∥ Unbiased assessment of study end points.
¶ Appropriateness of follow-up time after diagnosis.
# Inclusion of loss to follow-up.
⁎⁎ Prospective calculation of study size.
‡‡ Contemporary control groups.
§§ Baseline equivalence of groups on several factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


