Dabigatran is a novel oral anticoagulant and may be useful during atrial fibrillation (AF) ablation for prevention of thromboembolic events. However, the benefits and adverse effects of periprocedural dabigatran therapy have not been thoroughly evaluated. A meta-analysis was performed to evaluate the efficacy and safety of dabigatran for anticoagulation in AF ablation. PubMed, The Cochrane Library, EMBASE, Web of Science, and CINAHL databases were searched from January 01, 2001 through July 30, 2013. Two reviewers reviewed the studies for inclusion and extracted data from studies comparing dabigatran with warfarin for AF ablation. A total of 5,513 patients undergoing catheter ablation were included in 17 observational studies and 1 randomized trial. Fourteen events of stroke or transient ischemic attacks were reported in the dabigatran group and 4 in the warfarin group (Peto’s odds ratio 3.94, 95% confidence interval [CI] 1.54 to 10.08, number needed to harm = 284 patients). The risk of all thromboembolic complications was also higher in the dabigatran group compared with the warfarin group (Peto’s odds ratio 2.81, 95% CI 1.23 to 6.45). No major differences were observed for the risk of major bleeding (odds ratio 0.99, 95% CI 0.55 to 1.78), pericardial tamponade, and groin hematoma. A lower risk of minor bleeding was observed with dabigatran (odds ratio 0.60, 95% CI 0.41 to 0.87). In conclusion, periprocedural use of dabigatran for AF ablation was related to a higher risk of thromboembolic complications including stroke and transient ischemic attack.
Recently, the new oral anticoagulant dabigatran has been evaluated in randomized phase 3 trials and approved for use for nonvalvular atrial fibrillation (AF). Dabigatran in a dose of 150 mg twice daily showed superiority over warfarin for prevention of stroke or transient ischemic attacks (TIAs) and 110-mg twice-daily dose showed noninferiority for patients with AF. However, data supporting the use of dabigatran in patients with catheter ablation are lacking. Therefore, we performed a meta-analysis to evaluate the efficacy and safety of periprocedural dabigatran versus warfarin therapy in patients with AF undergoing catheter ablation.
Methods
We searched the published literature from January 1, 2001 through July 30, 2013 using the following key words: dabigatran, oral thrombin inhibitors, atrial fibrillation, and ablation. PubMed, The Cochrane Library (Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials), EMBASE, Web of Science, and CINAHL databases were searched. We restricted our search to reports published in English and studies of humans. We also manually searched reference lists of all retrieved reports for additional studies. We used the published strengthening Meta-analysis Of Observational Studies in Epidemiology checklist to select the studies for this review. We included published studies (full-text reports) and conference abstracts evaluating dabigatran versus warfarin for periprocedural catheter ablation for AF. We excluded studies without a comparator group, and for studies that did not report clinical outcomes, we tried to contact the individual corresponding authors for further details.
Two reviewers (RN and PS) independently extracted the data from the eligible studies using the standardized published protocol at PROSPERO, and disagreements were resolved by discussion with other investigators. We extracted the baseline characteristics of each study, details of the study drugs and control (drug, dose, and schedule), follow-up data, and the data related to efficacy and safety outcomes. The quality of the observational case-control studies was assessed by the Newcastle-Ottawa Scale, and for randomized controlled trials, we used the domains suggested in The Cochrane Handbook of Systematic Reviews. The primary efficacy outcome was stroke or TIA. The primary safety outcome was major bleeding. Other outcomes were the risk of all thromboembolic complications, minor bleeding, pericardial tamponade, and groin hematoma. We used the longest available follow-up data from individual studies for our analysis.
The statistical analysis was performed according to the recommendations from the Cochrane Collaboration using Review Manager, version 5.2 (The Nordic Cochrane Center, The Cochrane Collaboration, 2012, Copenhagen, Denmark). As outcome proportions were expected to be low, for outcomes with an incidence of ≤1%, we calculated Peto’s odds ratio (POR) estimates and associated 95% confidence intervals (CIs) using a fixed-effects model for our primary analyses. As previously validated, POR is one of the least biased and most powerful methods and provides the best CI coverage for event rates <1% and when treatment effects are not exceptionally large. For outcomes with event rates >1%, we used the conventional random-effects model and calculated the summary odds ratio (OR). We assessed heterogeneity across studies using the Cochrane Q statistic. For I 2 statistics, we considered I 2 <25% as low heterogeneity and I 2 >75% as high, and the Cochran Q (p ≤0.1) was considered significant for each outcome. Publication bias was evaluated using Egger’s regression test and through visual inspection of the asymmetry in funnel plots. Two-tailed p values <0.05 were considered significant. We calculated the number needed to harm (NNH)/number needed to treat (NNT) from POR using the following formula: NNH/NNT = 1/{CEP − [POR/(1/CEP − 1) + POR]}, where CEP = control event proportion.
We performed the study sequential analysis (SSA) with an intention to maintain an overall 5% risk of a type I error despite multiple testing and sparse data being standard in meta-analyses. We calculated our monitoring boundaries according to the required information size to detect or reject an intervention effect of a 150% POR increase to challenge risk ratios estimated from previous meta-analyses with a risk of a type II error of 20% (power of 80%). We also provide the 95% CIs adjusted for sparse data and repetitive testing, which we describe as the SSA-adjusted 95% CIs. We used TSA, version 0.9 beta ( www.ctu.dk/tsa ; Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark) for these analyses (details in the Supplementary Material , online only).
The following sensitivity analysis was performed for the primary efficacy and safety outcomes: (1) for studies published as full-text reports, (2) studies with low or intermediate risk of bias, (3) observational studies only, (4) studies with only dabigatran 150-mg twice-daily dose, (5) studies with dabigatran 110 mg twice daily, (6) prospective studies, (7) studies whose patients were treated with uninterrupted warfarin, (8) studies with at least 30-days follow-up, (9) studies with interrupted dabigatran, (10) studies with bridging low-molecular-weight heparin, and (11) studies conducted in the United States of America. Sensitivity analyses planned a priori were performed to identify the effect of a single study by sequential elimination of each study from the pool and assessing the overall outcomes. Stata 12 software was used (StataCorp LP, College Station, Texas).
Results
We identified 18 studies that satisfied our inclusion criteria ( Figure 1 ). We analyzed outcome data related to 5,513 patients. Baseline characteristics are listed in Supplementary Table 1 (online only). Of the identified observational studies, 11 were published in full-text reports, and 7 were conference abstracts. One small randomized trial was published from Japan. Most of the studies used dabigatran 150 mg twice daily as the most frequently used dose.
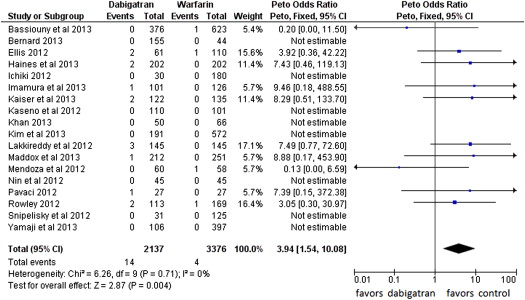
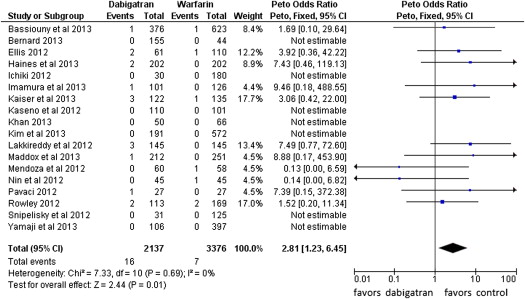
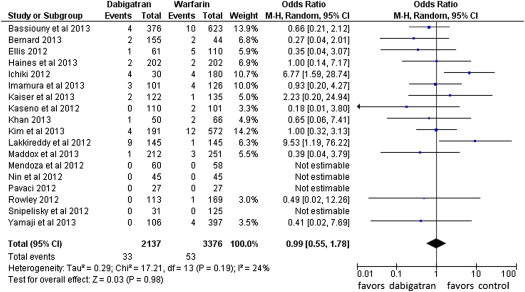
Fourteen events of stroke or TIA were reported in the dabigatran group (0.65%, of 2,137 patients) and 4 in the warfarin group (0.12% of 3,376 patients; POR 3.94, 95% CI 1.54 to 10.08, p = 0.004, NNH = 284 patients; Figure 2 ). The risk of all thromboembolic complications was also higher in the dabigatran group (16 events) compared with the warfarin group (7 events; 0.74% vs 0.21%, POR 2.81, 95% CI 1.23 to 6.45, p = 0.01, NNH = 264; Figure 3 ). No statistical heterogeneity was observed for any of these analyses. For stroke or TIA and all thromboembolic complications, the SSA revealed that the numbers of events were too few to conclude firmly for a 150% increase in POR ( Supplementary Figures 1 and 2 , online only). Thirty-three patients in the dabigatran group and 53 patients in the warfarin group had major bleeding (OR 0.99, 95% CI 0.55 to 1.78; Figure 4 ). A lower risk of minor bleeding was observed with dabigatran ( Supplementary Figure 3 , online only). The risk of pericardial tamponade observed with dabigatran was not significantly higher compared with warfarin ( Supplementary Figure 4 , online only). The increase of the risk of groin hematoma with dabigatran was also not significantly different compared with warfarin ( Supplementary Figure 5 , online only).
Only 150-mg twice-daily dabigatran (and 75 mg in selected patients) is approved in the United States, which is different from the rest of the world where 110-mg twice-daily dose is also approved and commonly prescribed. So a sensitivity analysis was performed with only United States studies. The results for stroke or TIA and major bleeding were consistent with our primary analysis. A significantly higher risk of stroke or TIA was observed with dabigatran compared with warfarin (POR 3.58, 95% CI 1 .32 to 9.70) in the United States population. A consistent high risk for stroke or TIA with dabigatran was observed in most of our important sensitivity analysis: studies published as full-text reports, studies with low or intermediate risk of bias, observational studies only, studies with follow-up of at least 30 days, studies with interrupted dabigatran therapy, and studies with bridging low-molecular-weight heparin ( Supplementary Table 2 , online only). Sensitivity analyses for major bleeding showed a comparable bleeding risk with dabigatran and warfarin in all subgroup analyses (data not shown). Sensitivity analyses by sequentially dropping each individual study and evaluating the overall outcomes failed to identify any of the individual studies as having influenced the outcomes to a significant extent, and the results were concordant with the overall analyses ( Supplementary Figure 6 , online only).
There was no evidence of small-study effects (publication bias) by visual inspection of funnel plots ( Supplementary Figure 7 , online only) and by Egger’s test.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


