The renin–angiotensin system is an important mediator of tumor progression and metastasis. A recent meta-analysis of randomized controlled trials reported an increased risk of cancer with angiotensin receptor blockers. It is unknown whether angiotensin-converting enzyme (ACE) inhibitors may have a similar effect. Our primary objective was to determine the effect of ACE inhibitors on cancer occurrence and cancer death. Our secondary objective was to determine the effect of ACE inhibitors on occurrence of gastrointestinal (GI) cancers given previous concerns of increased risk. Systematic searches of SCOPUS (covering MEDLINE, EMBASE, and other databases) and the Food and Drug Administration official web site were conducted for all randomized controlled trials of ACE inhibitors. Trials with ≥1 year of follow-up and enrolling a minimum of 100 patients were included. Fourteen trials reported cancer data in 61,774 patients. This included 10 trials of 59,004 patients providing information on cancer occurrence, 7 trials of 37,515 patients for cancer death, and 5 trials including 23,291 patients for GI cancer. ACE inhibitor therapy did not have an effect on occurrence of cancer (I 2 0%, risk ratio [RR] 1.01, 95% confidence interval [CI] 0.95 to 1.07, p = 0.78), cancer death (I 2 0%, RR 1.00, 95% CI 0.88 to 1.13, p = 0.95), or GI cancer (RR 1.09, 95% CI 0.88 to 1.35, p = 0.43). In conclusion, ACE inhibitors did not significantly increase or decrease occurrence of cancer or cancer death. There was also no significant difference in risk of GI cancer.
The renin–angiotensin system is a complex pathway critical to cardiovascular homeostasis. Drugs that antagonize this system, primarily angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are widely used for treatment of many cardiovascular conditions and for risk reduction. In recent years, it has become increasingly recognized that the renin–angiotensin system also plays an important role in cancer biology. For example, type 1 and type 2 angiotensin receptors are important regulators of cellular proliferation, angiogenesis, and inflammation. Although the exact role these receptors play in development of human cancer remains to be clarified, a recent meta-analysis of randomized clinical trials has reported that drugs directly blocking type 1 angiotensin receptors (i.e., ARBs) were associated with a modestly increased risk of cancer diagnosis. This publication inevitably led to the question of whether a closely allied class of drugs, ACE inhibitors, has a similar effect on cancer incidence. Therefore, we decided to perform a systematic meta-analysis of all randomized trials testing ACE inhibitors that reported cancer data. Our primary objective was to examine the effect of ACE inhibitors on cancer occurrence and cancer death. Because some previous clinical trials have reported an excess in gastrointestinal (GI) cancers with ACE inhibitors, our secondary objective was to determine effect of ACE inhibitors on occurrence of GI cancers.
Methods
We retrieved all randomized controlled trials reporting on ACE inhibitor therapy and human cancers published before December 2009. Electronic searches of SCOPUS (which includes MEDLINE, EMBASE, and several other databases from a wide range of disciplines) were supplemented with searches of the Food and Drug Administration web site. The search terms and other search strategies are described in detail in Appendix A (available online). Searches were limited to randomized controlled trials in human subjects reported in English. Links of every search result were examined thoroughly for any cancer information.
All search results were assessed for study duration and sample size. Because cancers usually have a long latency period and are relatively uncommon adverse events, randomized controlled trials were eligible for inclusion only if they had ≥1 year of follow-up and enrolled a minimum of 100 patients. These cut-off values of study duration and enrollment size were based on previous studies examining cancer outcomes. Only randomized controlled trials that were double blinded or had a prospective, randomized, open-label, blinded end-point design were included to decrease the possibility of confounding factors. Trials using active control or placebo control were included. However, trials comparing an ACE inhibitor to an ARB were excluded because ARBs have recently been reported to be associated with an increased cancer risk. Trials were also excluded if ACE inhibitors were used in all study arms.
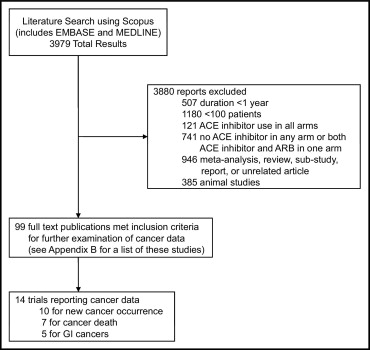
The literature search using SCOPUS yielded 3,979 results ( Figure 1 ). Ninety-nine trials that met the inclusion criterion were then examined in detail for any cancer data ( Appendix B , available online; Figure 1 ). Searches of Food and Drug Administration web site revealed cancer information from one trial. A total of 14 trials reported cancer information. Ten of these 14 trials reported new cancer occurrence, 7 reported cancer death, and 4 reported GI cancers.
Two investigators (J.C. and S.D.) independently extracted the data from the trials, which are presented in Tables 1, 2, and 3 .
| Study | Publication Year | Condition studied | Mean Follow-Up (years) | Total Number of Patients | Study Drug (number of patients) | Control (number of patients) | Mean Age (years) | Men | Black | Current Smoker | History of Cancer at Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Drug | Control | Study Drug | Control | Study Drug | Control | Study Drug | Control | Study Drug | Control | |||||||
| Trials with new cancer data and gastrointestinal cancer ⁎ data | ||||||||||||||||
| STOP-HTN-2 | 2001 | hypertension | 5.3 | 6,614 | lisinopril or enalapril upto 20 mg/day (n = 2,205) | β blocker, diuretic, or calcium channel blocker † (n = 4,409) | 76.1 | 76 | 33.7% | 33.0% | — | — | 9.4% ‡ | 8.8% ‡ | 9.4% | 9.0% |
| HOPE | 2000 | high cardiovascular risk | 5.0 | 9,297 | ramipril upto 10 mg/day (n = 4,645) | placebo (n = 4,652) | 66.0 | 66.0 | 72.5% | 74.2% | — | — | 13.9% | 14.5% | — | — |
| Maschio et al | 1996 | chronic renal insufficiency | 3.0 | 583 | benazepril 10 mg/day (n = 300) | placebo (n = 283) | 51.0 | 51.0 | 73.0% | 71.0% | — | — | — | — | 0 § | 0 § |
| SOLVD-P 7 | 1992 | asymptomatic systolic heart failure | 3.1 | 4,228 | enalapril upto 10 mg 2 times/day (n = 2,111) | placebo (n = 2,117) | 59.1 | 59.1 | 88.5% | 89.0% | 9.2% | 9.7% | 22.8% ‡ | 24.1% ‡ | 0 § | 0 § |
| SOLVD-T 6 | 1991 | symptomatic systolic heart failure | 3.5 | 2,569 | enalapril upto 10 mg 2 times/day (n = 1,285) | placebo (n = 1,284) | 60.7 | 61.0 | 80.9% | 79.8% | 16.2% | 14.5% | 22.8% | 21.4% | 0 § | 0 § |
| Trials with new cancer data and cancer death data | ||||||||||||||||
| PHARAO | 2008 | prehypertension | 3.0 | 1,008 | ramipril upto 5 mg/day (n = 505) | placebo (n = 503) | 62.2 | 62.3 | 49.7% | 47.1% | — | — | 12.1% ‡ | 16.7% ‡ | — | — |
| ALLHAT | 2002 | cardiovascular disease ¶ | 4.9 | 33,357 | lisinopril upto 40 mg/day (n = 9,054) | chlorthalidone upto 25 mg/day or amlodipine upto 10 mg/day (n = 24,303) | 66.9 | 66.9 | 53.8% | 52.9% | 35.5% | 35.3% | 21.9% ‡ | 21.9% ‡ | — | — |
| FACET | 1998 | noninsulin-dependent diabetes | 3.0 | 380 | fosinopril 20 mg/day (n = 189) | amlodipine 10 mg/day (n = 191) | 62.8 | 63.3 | 63.5% | 55.5% | — | — | 4.8% | 6.8% | — | — |
| Trials with new cancer data only | ||||||||||||||||
| PHYLLIS | 2004 | carotid atherosclerosis | 2.6 | 508 | fosinopril 20 mg/day (n = 255) | HCTZ 25 mg/day ± pravastatin (n = 253) | 58.5 | 58.3 | 40.0% | 40.5% | — | — | 12.3% | 20.1% | — | — |
| SCAT | 2000 | coronary artery disease | 4.0 | 460 | enalapril upto 10 mg 2 times/day (n = 229) | placebo (n = 231) | 60.0 | 62.0 | 89.0% | 89.0% | — | — | 15.0% | 15.0% | 0 § | 0 § |
| Trials with cancer death data only | ||||||||||||||||
| QUINS | 2007 | scleroderma | 3.0 | 210 | quinapril upto 80 mg/day (n = 104) | placebo (n = 106) | 54.0 | 55.0 | 16.0% | 13.0% | 5.0% # | 2.0% # | — | — | — | — |
| APRES | 2000 | coronary artery disease | 2.8 | 159 | ramipril upto 10 mg/day (n = 80) | placebo (n = 79) | 61.4 | 60.6 | 88.0% | 90.0% | — | — | 83.0% ‡ | 88.0% ‡ | — | — |
| SAVE | 1992 | asymptomatic systolic heart failure postmyocardial infarction | 3.5 | 2,231 | captopril upto 50 mg 3 times/day (n = 1,115) | placebo (n = 1,116) | 59.3 | 59.5 | 83.0% | 82.0% | — | — | 53.0% | 53.0% | — | — |
| MMHF | 1992 | systolic heart failure | 2.7 | 170 | captopril upto 25 mg 2 times/day (n = 83) | placebo (n = 87) | 62.4 ⁎⁎ | 62.4 ⁎⁎ | 75.0% ⁎⁎ | 75.0% ⁎⁎ | — | — | — | — | 0 § | 0 § |
⁎ See Appendix C for individual trial gastrointestinal cancer data (available online).
† Beta blocker = atenolol upto 50 mg/day, metoprolol upto 100 mg/day, or pindolol upto 5 mg/day; diuretic = hydrochlorothiazide upto 25 mg/day or amiloride upto 2.5 mg/day; calcium channel blocker = felodipine upto 5 mg/day or isradipine upto 5 mg/day.
‡ Includes previous and current smoking.
§ Patients with cancer history excluded from study.
¶ Defined as coronary artery, peripheral vascular, or cerebrovascular disease.
# Percentage of nonwhite participants.
⁎⁎ Percentages of angiotensin-converting enzyme inhibitor and control groups combined; data not available according to study groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


