Because carvedilol is a unique vasodilating β blocker (BB) exerting antioxidant activity and pleiotropic effects, it was theorized that it may confer more potent beneficial effects on cardiovascular mortality and morbidity in acute myocardial infarction (AMI) and heart failure (HF) settings. A systematic review and meta-analysis was performed of randomized, controlled, direct-comparison trials that included adults receiving atenolol, bisoprolol, metoprolol, nebivolol, or carvedilol to evaluate the effects of carvedilol compared to other BBs on mortality, cardiovascular events, and hospital readmissions in the setting of AMI or systolic HF. Compared to β 1 -selective BBs used in HF (8 trials, n = 4,563), carvedilol significantly reduced all-cause mortality (risk ratio 0.85, 95% confidence interval 0.78 to 0.93, p = 0.0006). In 3 trials of patients with AMI (n = 644), carvedilol significantly reduced all-cause mortality by 45% (fixed-effects model: risk ratio 0.55, 95% confidence interval 0.32 to 0.94, p = 0.03, random-effects model: risk ratio 0.56, 95% confidence interval 0.26 to 1.12, p = 0.10), with no reduction in non-fatal MI (risk ratio 0.61, 95% confidence interval 0.31 to 1.22, p = 0.16). In conclusion, carvedilol, as compared against atenolol, bisoprolol, metoprolol and nebivolol in randomized direct comparison trials, significantly reduced all-cause mortality in systolic HF patients. Additionally, carvedilol significantly reduced all-cause mortality compared with β 1 -selective BBs in AMI patients using the fixed-effects model but not using the random-effects model.
Although guidelines for the treatment of patients with acute coronary syndromes, acute myocardial infarctions (AMIs), and heart failure (HF) recommend the use of β blockers (BBs) as first-line therapy, they do not specifically recommend 1 BB over another. However, carvedilol has been shown to significantly reduce all-cause mortality and cardiovascular (CV) events (nonfatal and fatal stroke and myocardial infarction [MI]) compared to metoprolol in patients with HF (in the Carvedilol or Metoprolol European Trial [COMET]). Thus, we sought to compare carvedilol against the most frequently prescribed β 1 -selective BBs (atenolol, metoprolol, and bisoprolol) and the most recently approved β 1 -selective BB (nebivolol) in patients with AMI or systolic HF.
Methods
A systematic review of the available published research according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for the conduct of systematic reviews of intervention studies was performed. Studies were identified through searches of the following sources: Ovid MEDLINE (1977 to 2012), PubMed (1978 to 2011), and Embase (1977 to 2012). To identify further potentially relevant studies missed by the electronic database search, reference lists from identified trials and review reports were manually screened. Searches were restricted to the English language and were updated using automated weekly e-mail alerts until August 2012. Full details of the search strategies and excluded trials are available as appendices by request.
Studies were selected for inclusion on the basis of the following criteria: (1) study design: randomized controlled trials, (2) type of participants: adults (aged ≥18 years), (3) intervention: carvedilol, (4) comparator: atenolol, bisoprolol, metoprolol, or nebivolol, and (5) outcomes: all-cause mortality, CV events (fatal and nonfatal strokes, fatal and nonfatal MI), and HF or CV-related hospital readmissions. We excluded studies that did not report mortality or morbidity outcomes. The titles and abstracts of studies identified by the search strategy were independently screened by 2 reviewers (J.J.D. and H.F.), and clearly irrelevant studies were discarded.
The following data elements were extracted from each study: the number of patients per arm, the nature of the intervention, patient inclusion criteria, cause of HF, type of AMI index event (i.e. percentage non–ST-segment elevation MI, percentage ST-segment elevation MI, percentage unstable angina), and baseline and follow-up blood pressure, ejection fraction, and heart rate ( supplemental tables are available by request). The following outcomes were also extracted from each trial: all-cause mortality, CV events (nonfatal and fatal stroke, nonfatal and fatal MI), and HF or CV-related hospital readmissions. Quality assessment was judged according to the following criteria: concealment of treatment allocation; similarity of the 2 groups at baseline regarding prognostic factors and medication use; blinding of outcome assessors, care providers, and patients; completeness of follow-up; and intention-to-treat analysis ( supplemental tables are available by request). Overall study quality was quantified using the Jadad score. The data extraction and quality assessment was undertaken using a standardized pro forma by 2 reviewers (H.F. and A.R.M.).
We express outcome results for each study as risk ratios (RRs) with 95% confidence intervals (CIs). Summary estimates were computed using a DerSimonian and Laird fixed and random-effects and fixed-effects meta-analysis model. We report pooled results as RRs and numbers needed to treat. Statistical heterogeneity across trials was estimated using the I 2 statistic, where I 2 <30% denotes low heterogeneity, I 2 = 30% to 50% represents moderate heterogeneity, and I 2 >50% denotes substantial heterogeneity. Two-tailed p values <0.05 were considered statistically significant for all analyses. Cochrane Review Manager version 5 software was used for all analyses. A sensitivity analysis was conducted to account for different BBs in the direct-comparison trials against carvedilol.
Results
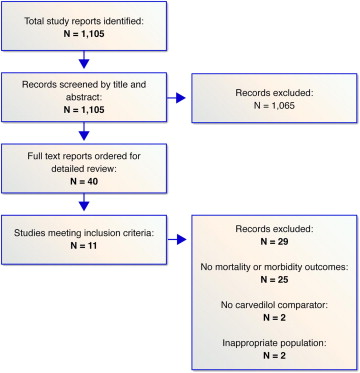
The search of the published research yielded 1,105 titles, of which 40 were reviewed in full text on the basis of the inclusion criteria ( Figure 1 ). Of these, 11 studies were deemed eligible for inclusion ( Figure 1 ). Tables containing the characteristics of the included studies are available by request.
All trials were randomized direct comparison trials against carvedilol in patients with either HF or AMI. All background medications and baseline characteristics were statistically similar between the comparison groups in each trial, except for 3 trials: Mrdovic et al had significantly more patients with hyperlipidemia at baseline in the carvedilol group and older, more Killip class IV HF patients in the metoprolol group; Jonsson et al had significantly more atenolol patients being female and receiving intravenous diuretics during treatment; and Marazzi et al had significantly more baseline coronary artery disease or idiopathic dilated cardiomyopathy patients and higher baseline heart rates in the carvedilol group but higher baseline systolic blood pressures and lower ejection fractions in the nebivolol group. Trials enrolled a median of 150 patients (interquartile range 67 to 3.029) with a median follow-up period of 12 months (interquartile range 0.5 to 58).
Four studies scored well on the methodologic quality indicators ( supplemental tables with details are available by request). Concealed allocation and blinding of ≥1 outcome assessment were stated in 3 and 5 of 11 trials, respectively.
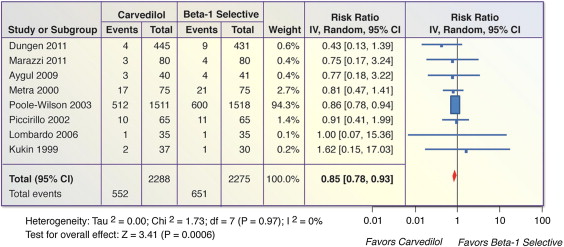
Eight trials (n = 4,563) reported on all-cause mortality in patients with systolic HF. There was a significant decrease in mortality for carvedilol compared to β 1 -selective BBs (RR 0.85, 95% CI 0.78 to 0.93, p = 0.0006, I 2 = 0%; Figure 2 ). The number needed to treat over the course of the trials was 22 (95% CI 14 to 52).
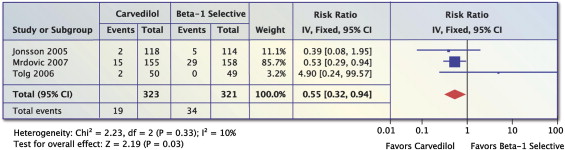
Three trials (n = 644) reported on all-cause mortality in patients with AMI. There was a significant 45% reduction in all-cause mortality in patients on carvedilol vs. β 1 -selective BBs by using a fixed-effects model (RR 0.55, 95% CI 0.32 to 0.94, p = 0.03, Figure 3 ) but not by random-effects model (RR 0.56, 95% CI 0.26 to 1.12, p = 0.10), I 2 = 10%. The number needed to treat over the course of the trials was 21 (95% CI 11 to 227).
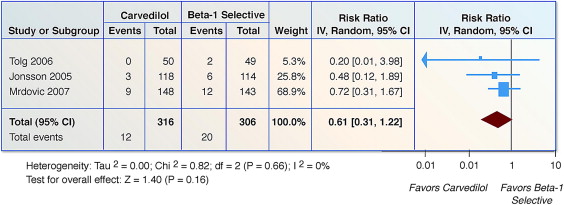
Three trials (n = 644) reported on nonfatal MI. For patients taking carvedilol compared to β 1 -selective BBs, there was no reduction in nonfatal MI (RR 0.61, 95% CI 0.31 to 1.22, p = 0.16, I 2 = 0%; Figure 4 ).
Two trials (n = 3,099) reported on HF readmissions. Compared to β 1 -selective BBs, there was no significant benefit on reducing HF readmissions with carvedilol (RR 0.98, 95% CI 0.93 to 1.03, p = 0.45) I 2 = 0%; ( Figure 5 ).
Only 1 trial (n = 232) compared atenolol to carvedilol in patients with AMI. Compared to atenolol, carvedilol did not reduce all-cause mortality, although the small sample size may have rendered this nonsignificant (RR 0.39, 95% CI 0.08 to 1.95, p = 0.25).
Two trials in patients with HF (n = 957) were performed with bisoprolol versus carvedilol. Compared to bisoprolol, carvedilol did not reduce all-cause mortality (RR 0.54, 95% CI 0.22 to 1.34, p = 0.19, I 2 = 0%).
Four HF trials compared metoprolol tartrate to carvedilol (n = 3,376). Compared to metoprolol, carvedilol caused a significant reduction in all-cause mortality (RR 0.86, 95% CI 0.78 to 0.94, p = 0.001, I 2 = 0%). In the AMI setting, 2 direct-comparison trials (n = 390) indicated no significant reduction in all-cause mortality with carvedilol compared to metoprolol tartrate (RR 0.95, 95% CI 0.13 to 7.13, I 2 = 53%). However, the study by Mrdovic et al, carrying approximately 86% of the weight for AMI mortality end points, indicated that carvedilol was associated with a significant 47% reduction in all-cause mortality (RR 0.53, 95% CI 0.29 to 0.94, p = 0.02).
Two HF trials compared nebivolol to carvedilol (n = 230). Compared to nebivolol, carvedilol was not associated with a reduction in all-cause mortality (RR 0.80, 95% CI 0.22 to 2.91, p = 0.73, I 2 = 0%).
Results
The search of the published research yielded 1,105 titles, of which 40 were reviewed in full text on the basis of the inclusion criteria ( Figure 1 ). Of these, 11 studies were deemed eligible for inclusion ( Figure 1 ). Tables containing the characteristics of the included studies are available by request.




