Rosuvastatin and atorvastatin both are high-intensity statins. However, which statin is more effective for the reversion of coronary atherosclerotic plaques remains inconclusive. We, therefore, conducted a meta-analysis to provide further evidence for proper statin selection. Pubmed, The Cochrane Library, Embase, Chinese BioMedicine, and China National Knowledge Infrastructure databases were systematically searched for eligible publications. We also manually reviewed the references from all relevant literature for more trials. Only studies that met our predefined inclusion criteria up to March 31, 2015, were enrolled. Five randomized controlled trials, 4 published in English and 1 in Chinese, were finally included in our study with a total of 1,556 participants, of whom 772 were in the rosuvastatin group and 784 in the atorvastatin group. The dose ratios of rosuvastatin versus atorvastatin were 1:2 in all included trials. Pooling across the studies demonstrated that compared with atorvastatin, rosuvastatin administration further reduced the total atheroma volume (weighted mean difference [WMD] −1.61 mm 3 , 95% confidence interval [CI] −2.70 to −0.52; p = 0.004) and percent atheroma volume (WMD −0.34%, 95% CI −0.64 to −0.03; p = 0.03) and improved the lumen volume more significantly (WMD 2.10 mm 3 , 95% CI 0.04 to 4.17; p = 0.046). The comparative regression of plaques was not different across subgroups. In conclusion, rosuvastatin is superior to atorvastatin in the reversion of coronary atherosclerotic plaques.
Coronary artery atherosclerosis (CAAS) is a crucial pathophysiological basis of coronary heart disease, and the unstability and rupture of atherosclerotic plaques result in acute coronary syndrome. Recent studies demonstrated that changes in plaque volume evaluated by intravascular imaging, such as intravascular ultrasound (IVUS), may represent a surrogate for adverse cardiovascular events in patients with CAAS. In addition to the reduction of low-density lipoprotein cholesterol (LDL-C) levels, statin also shows benefit in anti-inflammation, anti-thrombosis, and regulation of vascular endothelial function. A number of clinical trials have indicated that statin therapy can improve the composition of plaques and delay or even reverse the progression of plaques. Rosuvastatin and atorvastatin are the 2 most commonly used statins in clinical practice. However, inconsistent results were reported in trials comparing the effect of these 2 statins on the regression of coronary atherosclerotic plaques, thus performing a meta-analysis that is needed to provide reliable evidence for optimal statin selection.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. We performed systematic literature searches in the databases of PubMed, The Cochrane Library, Embase, Chinese BioMedicine, and China National Knowledge Infrastructure for identifying publications. The reference lists of relevant studies and review articles were also checked to find additional citations. We combined the following search terms: “rosuvastatin,” “atorvastatin,” “plaque” or “atherosclerosis” or “atheroma,” and “intravascular ultrasound” or “IVUS.” Our search was restricted to English and Chinese reports published until March 31, 2015.
Clinical studies that met the following conditions were included: (1) randomized controlled trials (RCTs) comparing rosuvastatin with atorvastatin in terms of regressing coronary atherosclerotic plaques, (2) with a sample size ≥30 and follow-up duration ≥3 months, (3) changes in plaque volume were measured by IVUS, and (4) reporting at least one of the following data: total atheroma volume (TAV), percent atheroma volume (PAV), and the lumen volume (LV). Post hoc analyses of the included RCTs were excluded.
In total, 2 reviewers independently abstracted the details regarding study and patient characteristics, treatment strategy, and follow-up duration. The total number of clinical end points and effect sizes (weighted mean difference [WMD]) were also recorded for the pooled analysis. If possible, we contact the corresponding authors of primary studies for missing data. The quality of the included articles was evaluated according to the Cochrane collaboration’s tool for assessing the risk of bias. Discrepancies were resolved by discussion with a third reviewer.
Data analysis was conducted using STATA 12.0 (StataCorp, College Station, Texas). The WMD of changes with its 95% confidence intervals (CIs) for all outcomes were used to present the pooled effect size of individual studies. Statistical heterogeneity across the studies was investigated by the Cochrane Q test with a significant level of p <0.1 and quantified by the I 2 statistic. When I 2 <50% (indicate no significant heterogeneity), we reported a pooled estimate under the fixed-effects model, otherwise the random-effects model was used. Subgroup analysis was performed to assess the influence of race, follow-up duration, statin dose, and comparative LDL-C reduction on the pooled estimates. Differences between subgroups were confirmed with the heterogeneity test based on the notion of performing a test for heterogeneity across subgroups rather than across studies. The results of pooled effect size and subgroups analyses were considered to be statistically significant only at p <0.05.
Results
The study selection process is displayed in Figure 1 . Briefly, 56 of the initial 66 citations were excluded after scanning the titles and/or abstracts. The remaining 10 articles were selected for full-text screening, during which 5 publications did not meet the inclusion criteria. Consequently, 5 RCTs that were performed from 2008 to 2012 were included in our meta-analysis.
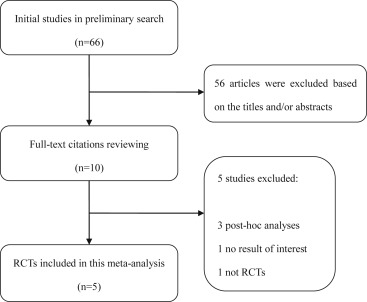
Table 1 summarizes the baseline characteristics of the eligible studies. All of them were designed using the randomized controlled method with a 1:2 dose ratio of rosuvastatin versus atorvastatin. A total of 1,556 participants with CAAS were enrolled, of whom 772 were assigned to the rosuvastatin group and 784 to the atorvastatin group. The average age ranged from 57 to 61 years, and 76% of the participants were men. The mean duration of follow-up ranged from 3 to 26 months. Baseline serum lipid profiles were not significantly different across the enrolled studies. Moreover, only the SATURN (Study of coronary Atheroma by InTravascular Ultrasound: effect of Rosuvastatin vs atorvastatiN) trial was supported by a company marketing rosuvastatin (AstraZeneca; AstraZeneca Pharmaceuticals, London, United Kingdom). Outcomes in the included studies were listed in Table 2 .
| Study | N | Intervention | Age (years) | Male n (%) | Baseline lipid profile | Follow-up (months) | |||
|---|---|---|---|---|---|---|---|---|---|
| Rosuvastatin (mg/d) | Atorvastatin (mg/d) | TC (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) | |||||
| Hong 2008 | 30 | 20 | 40 | 61 | 18 (60) | 180.9 | 123.8 | 49.2 | 12 |
| Hong 2011 | 128 | 20 | 40 | 58 | 95 (74) | 183.0 | 119.5 | 47.5 | 11 |
| Nicholls 2011 | 1039 | 40 | 80 | 58 | 765 (74) | 193.7 | 120 | 45 | 26 |
| Guo 2012 | 88 | 10 | 20 | 59 | 77 (88) | – | 113.3 | 36.3 | 3-6 |
| Lee 2012 | 271 | 10 | 20 | 57 | 223 (82) | 184.4 | 109.5 | 40 | 6 |
| Study | TAV (mm 3 ) | PAV (%) | LV (mm 3 ) | |||
|---|---|---|---|---|---|---|
| Rosuvastatin | Atorvastatin | Rosuvastatin | Atorvastatin | Rosuvastatin | Atorvastatin | |
| Hong 2008 | -5.62±7.71 ∗ | -4.71±8.51 | -0.80±1.27 | -0.57±1.15 | 3.68±5.86 | 2.00±6.61 |
| Hong 2011 | -4.4±7.3 ∗ | -3.6±6.8 | -0.73±2.05 | -0.19±2.10 | 1.3±7.1 | -1.0±7.3 |
| Nicholls 2011 | -6.39±13.96 † | -4.42±15.81 | -1.22±3.61 ∗ | -0.99±3.65 | – | |
| Guo 2012 | -5.68±5.11 | -4.46±3.4 | – | – | ||
| Lee 2012 | -18.78±26.04 ∗ † | -9.93±28.02 | -1.0±3.5 | -0.3±4.2 | – | |
∗ Primary outcome in each trial.
Overall, random sequence generation was observed in 2 studies, and both of them reported allocation concealment. One trial was double blinded, and 2 studies performed blinded assessments of the outcomes. Neither incomplete outcome data nor selective data report was present in any of the included studies. Additionally, we did not find other significant bias during the quality evaluation ( Supplementary Figure 1 ).
All included studies reported the changes from baseline to the end of follow-up in TAV, and no evidence of heterogeneity was observed across them (I 2 = 30%, p = 0.223). The pooled estimate using fixed-effect meta-analysis showed a significantly greater reduction in TAV for the patients treated with rosuvastatin (n = 772) compared with those treated with atorvastatin (n = 784; WMD −1.61 mm 3 , 95% CI −2.70 to −0.52, p = 0.004; Figure 2 ). Four trials reported the data of PAV without obvious heterogeneity across them (I 2 = 0%, p = 0.742). For this outcome, 729 participants received rosuvastatin therapy, whereas 739 patients received atorvastatin treatment. Pooling across the 4 trials using fixed-effect meta-analysis revealed a more pronounced regression of PAV in the rosuvastatin group compared with the atorvastatin group (WMD −0.34%, 95% CI −0.64 to −0.03, p = 0.03; Figure 2 ).
Only 2 studies provided data for the LV, and statistical heterogeneity across them was not significant (I 2 = 0%, p = 0.784). Meta-analytic pooling for this outcome demonstrated that compared with the atorvastatin administration, rosuvastatin administration resulted in a statistically greater increase in the LV (WMD 2.10 mm 3 , 95% CI 0.04 to 4.17, p = 0.046; Supplementary Figure 2 ).
For better understanding of the association between different statins and regression of coronary plaque, we further analysed the data on LDL-C reduction, high-density lipoprotein cholesterol increase, and high-sensitive C-reactive protein reduction. Pooling across all available studies under a fixed-effect model demonstrated that rosuvastatin had more pronounced effect on reducing LDL-C than atorvastatin (p <0.001) without difference in increasing high-density lipoprotein cholesterol (p = 0.22) and reducing high-sensitive C-reactive protein (p = 0.68; Supplementary Figures 3 to 5 ).
For the reduction in TAV, we found no heterogeneity across the differences in races (p = 0.63), follow-up durations (p = 0.74), statin dosages (p = 0.80), and comparative LDL-C reduction (p = 0.26; Figure 3 ). Similar results were observed for the reduction in PAV ( Figure 4 ).




