Chapter 151
Mesenteric Vascular Disease
General Considerations
Ruby C. Lo, Marc L. Schermerhorn
Based on a chapter in the seventh edition by Juan Carlos Jimenez and William J. Quinones-Baldrich
Mesenteric ischemia occurs when perfusion of the visceral organs fails to meet normal metabolic requirements. This disorder is categorized as either acute or chronic on the basis of the duration of symptoms. Acute mesenteric ischemia (AMI) occurs rapidly during hours to days and frequently leads to acute intestinal infarction requiring resection (see Chapter 153). The most common causes are embolization to the mesenteric arteries and acute thrombosis related to a preexisting plaque. Chronic mesenteric ischemia (CMI) is a more insidious process and progresses during weeks to several months (see Chapter 152). The most common cause is progressive occlusive disease of the visceral arteries, usually related to atherosclerosis. Because CMI is relatively uncommon, it is frequently misdiagnosed as a gastrointestinal disorder. Patients typically have undergone an extensive workup for other potential causes before being diagnosed with CMI.
The first case of AMI was diagnosed and treated successfully with intestinal resection and reanastomosis by Elliott in 1895. Goodman first described chronic intestinal angina as a clinical disorder in 1918.1 Dunphy, a surgical resident at the Peter Bent Brigham Hospital, in 1936 reported a case of a patient with weight loss and pain out of proportion to abdominal findings who subsequently died and was found to have mesenteric occlusive disease on autopsy. After reviewing the medical records of 12 patients who died of intestinal angina, he found that 7 of the 12 (58%) had a history of chronic abdominal pain, thus introducing the potential for early intervention to prevent disease progression and death.2 Warren and Eberhard were the first to describe mesenteric venous thrombosis (MVT) as a distinct cause of intestinal infarction in 1935, differentiating it from occlusion of the mesenteric arteries.3
In the modern era, Klass4 performed the first superior mesenteric artery (SMA) embolectomy in 1950, avoiding intestinal resection. Although the patient died several days later of a heart-related condition, autopsy revealed the bowel to be normal. In 1958, Shaw and Maynard5 performed the first successful thromboendarterectomy of the SMA at the Massachusetts General Hospital. Morris et al6 performed the first successful retrograde bypass graft from the infrarenal aorta to the SMA in 1962. Stoney and Wylie7 at the University of California, San Francisco, first described antegrade aortovisceral bypass and transaortic visceral thromboendarterectomy in 1966. Ushering in the current era of percutaneous treatment of visceral arterial occlusive disease, Furrer8 and Novelline9 separately published the initial reports of endovascular dilatation of the SMA in 1980, and Finch10 first reported the treatment of a celiac artery stenosis with a Palmaz stent.
Anatomy of The Visceral Arteries
The primitive dorsal aorta gives rise to the abdominal aorta during fetal development. Ventral segmental arteries emerge from the primitive ventral aorta, which disappears around the fourth week of gestation. Multiple segmental branches from the primitive ventral aorta—the 10th, 13th, and 21st—persist and develop into the celiac artery, SMA, and inferior mesenteric artery (IMA), respectively. Disparity in the regression of the primitive ventral aorta and its segmental branches infrequently causes deviations in the visceral arterial anatomy.
The celiac artery arises from the abdominal aorta just caudal to the diaphragm at the level of L1 and is bordered by the median arcuate ligament at the aortic hiatus superiorly and the superior border of the pancreas inferiorly. Traditionally, the three branches from this common trunk include the left gastric, splenic, and common hepatic arteries. However, multiple variations of the true “trifurcation” can exist. Most frequently, the common hepatic artery and its branches arise from the SMA or directly from the abdominal aorta.
Exposure of the celiac trunk is best achieved through a midline transabdominal incision, which also allows visual assessment of bowel viability during surgical revascularization. The celiac trunk and its branches are surrounded by the celiac plexus of nerves, which must be divided for proximal exposure. A midline laparotomy is performed, and the triangular ligament is divided. The gastrohepatic ligament is then divided longitudinally to the level of the posterior parietal peritoneum. The liver is carefully retracted to the right of midline with a self-retaining retractor. Placement of a nasogastric tube facilitates identification of the esophagus. The posterior peritoneum overlying the diaphragmatic crus is divided sharply to expose the celiac trunk. The first vessel encountered is usually the common hepatic artery traversing to the right of midline toward the liver. The hepatic artery can be exposed back to the origin of the celiac trunk, which is covered by lymphatic tissue and the celiac nerve plexus. The diaphragmatic crus is divided to expose the celiac origin and the supraceliac aorta.
The SMA arises a few centimeters caudal to the celiac trunk, and its origin is crossed by the neck of the pancreas and the splenic vein. It arises at a less acute and downward-sloped angle than the celiac trunk and lies superior to both the uncinate process of the pancreas and the third portion of the duodenum. The superior mesenteric vein runs parallel adjacent to the artery, usually along its right border. The first important branch of the SMA is usually the inferior pancreaticoduodenal artery, which supplies collateral circulation with the celiac artery through the gastroduodenal and superior pancreaticoduodenal arteries. The second major branch of the SMA is frequently the middle colic artery, which arises at the inferior border of the pancreas. The right colic, ileocolic, and third-order mesenteric branches arise distally, supplying the small bowel within the mesentery.
During surgical exposure, the SMA can be approached either anteriorly at the base of the transverse colon mesentery or lateral to the fourth portion of the duodenum. Anterior SMA exposure involves lifting the transverse colon superiorly to clearly expose the base of its mesentery. The small intestine is covered in a moist towel or a bowel bag and retracted to the right. A horizontal incision is made through the posterior peritoneum at the base of the mesentery at the level of the proximal jejunum and extended to the right of midline. The middle colic artery can be used as a landmark within the transverse colon mesentery and to localize the main SMA trunk. Palpation often aids in localizing the SMA. The superior mesenteric vein is often visualized first, and the SMA can be palpated adjacent and to the left of it. This approach provides excellent exposure of the SMA. Exposure of the more proximal SMA to the left and lateral to the fourth portion of the duodenum can also be achieved. The ligament of Treitz is divided, and the lateral wall of the duodenum is mobilized off the anterior surface of the aorta. The SMA can be identified just distal to its origin from the aorta.
The IMA is usually located 3 to 4 cm cephalad to the aortic bifurcation, just to the left of midline, and usually arises at the level of the third lumbar vertebra. The main trunk frequently divides into sigmoidal branches and the left colic artery. The ascending left colic artery forms the inferior marginal artery of Drummond, which is the major collateral arcade between the SMA and IMA. The SMA and IMA are also linked by the meandering mesenteric artery (of Moskowitz), known historically as the arc of Riolan. This vessel runs more centrally, medial to the mesenteric border of the colon and through the middle of the mesenteric arcade near the inferior mesenteric vein. The meandering mesenteric artery is likely to be produced by the dilation of a normal collateral vessel in response to significant stenosis or occlusion of the SMA or IMA.11 Sigmoidal branches lead to the left and right superior rectal arteries, which collateralize with branches of the hypogastric arteries in the pelvis.
Physiology of Splanchnic Blood Flow
Normal intestinal function and nutrient absorption rely on adequate perfusion and oxygenation to the microvascular splanchnic circulation. Various autoregulatory mechanisms ensure adequate gut circulation through both vasoconstriction and relaxation of arterial smooth muscle. The degree of visceral artery dilatation and constriction determines the relatively large fluctuations in splanchnic blood flow during fasting, postprandial states, and periods of extreme stress. Visceral blood flow can vary dramatically, ranging from 10% of cardiac output in the setting of shock or hypovolemia, to 20% to 25% at rest or while fasting, and up to 35% after a large carbohydrate meal.12,13 Seventy percent to 80% of mesenteric blood flow supplies the mucosal and submucosal layers. The severely diminished blood flow observed in patients with nonocclusive mesenteric ischemia results from severe vasospasm related to this process. Duplex studies demonstrate moderate to high arterial resistance in the SMA circulation, with low diastolic flow and slight flow reversal during fasting states. In the postprandial period, low-resistance signals are noted throughout both systole and diastole, indicative of dilated splanchnic arteriolar beds; flow reversal does not occur. In contrast, low arterial resistance signals are noted in the celiac artery circulation regardless of feeding, probably because of the influence of the low-resistance hepatic vascular bed. Perko et al14 also noted that in fasting subjects performing submaximal exercise, splanchnic vascular resistance doubled and exhibited a 50% reduction in hepatosplenic blood flow and a 25% reduction in mesenteric blood flow.
Multiple mechanisms are responsible for regulating mesenteric arteriolar smooth muscle tone, and they are often interdependent. Extrinsic factors include sympathetic efferent nerves in the prevertebral celiac and mesenteric ganglia, which initiate stimuli for arterial vasoconstriction. Hormonal pathways also contribute to extrinsic regulation of splanchnic blood flow. The renin-angiotensin feedback mechanism causes mesenteric vasoconstriction through the direct action of angiotensin II during hypovolemic states. Low-volume states and hyperosmolarity stimulate the neurohypophysis, which releases vasopressin,15 a hormone that causes splanchnic vasoconstriction, reduction in portal venous pressure, and venodilatation.15 In addition, when subjected to shear stress, activation of the nitric oxide synthase enzymes on the surface of red blood cell membranes occurs, leading to significant dilation of mesenteric arteries under hypoxic conditions.16
Intrinsic regulation also occurs through metabolic and myogenic feedback mechanisms.15 In the metabolic pathway, mucosal ischemia prompts the release of metabolic byproducts, causing vasodilatation in arteriolar smooth muscle and preferentially shunting increased blood flow to the intestinal mucosa. In the myogenic pathway, which dominates the regulation of blood flow in the small intestines,17 abrupt decreases in perfusion pressure are sensed by arteriolar baroreceptors, which respond by decreasing arteriolar wall tension to maintain blood flow.18 Together, these mechanisms maintain mucosal perfusion and integrity during periods of relative ischemia.
Epidemiology
Asymptomatic occlusive disease of the visceral arteries is a common finding in elderly patients. Wilson et al15 demonstrated that 17.5% of 553 consecutive patients older than 65 years examined with duplex ultrasonography (DUS) had a critical stenosis of at least one visceral vessel. In addition, autopsy studies have estimated the prevalence of atherosclerosis involving the mesenteric arteries to be between 6% and 10%.19
Despite an aging population, hospitalizations for AMI in the United States have declined from 9.6 to 6.7 per 100,000 from 1998 to 2010.20 There is some evidence that this decline may be related to the increasing and widespread use of statins and the efficacy of warfarin in the prevention of thromboembolic events in patients with atrial fibrillation.21 Probably in part because of differences in longevity, AMI and CMI disproportionately affect women, with a female-to-male ratio of approximately 3 : 1.22
Because the majority of patients with mesenteric occlusive disease manifest no symptoms, the exact incidence of CMI is not known. However, admissions for CMI account for less than 1 per 100,000 admissions23 and have been increasing steadily in recent years in the United States.22 Whether these figures represent an actual increasing incidence or simply increased re-intervention due to restenosis from endovascular therapy remains to be determined. Despite the high prevalence of individuals with asymptomatic mesenteric arterial occlusive disease, patients usually demonstrate involvement of two or more mesenteric vessels before symptoms arise owing to the development of extensive collateralization over time. In fact, in a study by Thomas et al,24 who observed 980 consecutive patients with asymptomatic significant (50%) stenosis of at least one mesenteric artery, only four patients developed mesenteric ischemia and all of them had significant three-vessel disease after follow-up of 1 to 6 years. The variability of symptoms in patients with chronic abdominal pain often makes the diagnosis challenging, resulting in treatment delays and increased morbidity.
Pathophysiology
Chronic Mesenteric Ischemia
Atherosclerosis is the most common cause of CMI, and patients frequently have a history of smoking, hypertension, and hyperlipidemia. They may also have evidence of atherosclerotic disease in other vascular beds, particularly coronary, cerebrovascular, renal, aortoiliac, and other peripheral arteries. Although it is much more uncommon, CMI may also be seen in association with vasculitis and other inflammatory conditions, such as lupus, Buerger’s disease, and radiation arteritis. Median arcuate ligament syndrome is a separate entity that may lead to symptoms of CMI and is caused by the compression of the celiac artery by the median arcuate ligament. Symptoms are classically exacerbated by full expiration.
Acute Mesenteric Ischemia
Embolism
Arterial emboli are the most common cause of AMI, representing 40% to 50% of cases.25 The proximal source of the embolus is frequently intracardiac mural thrombus that develops in patients with atrial tachyarrhythmias, myocardial infarction, cardiomyopathy, structural heart defects, and cardiac tumors. Endocarditis can result in septic emboli from affected valve leaflets. Mural thrombus in proximal aneurysms in the thoracic or proximal abdominal aorta can also serve as an embolic source, as can atheromatous plaque even in the absence of aneurysm. The SMA is the most common final destination for mesenteric emboli, perhaps because of its relative size and the decreased angle of takeoff from the abdominal aorta compared with the other mesenteric vessels. In addition, such emboli tend to lodge several centimeters from the vessel’s origin, usually distal to the middle colic artery (Fig. 151-1A). The angiographic hallmark of an embolic occlusion seen on computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is an abrupt cutoff just beyond the origin of the middle colic artery.26
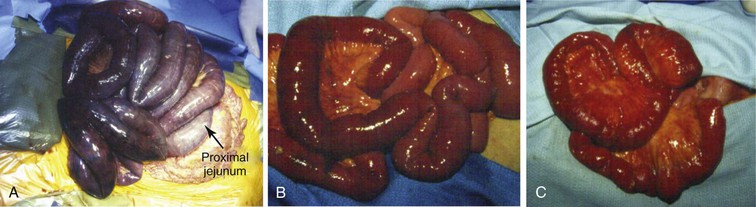
Figure 151-1 A, Operative findings typical of acute mesenteric ischemia secondary to an embolus. Note that the first portion of the jejunum is spared because the embolus lodged distal to the middle colic artery. B, Operative findings typical of acute mesenteric ischemia secondary to arterial thrombosis. Note that the entire bowel is affected. C, Appearance of the small bowel at second-look surgery after revascularization. Note the hemorrhagic changes in the mesentery.
Arterial Thrombosis
Arterial thrombosis constitutes the next most common cause of AMI and occurs in 20% to 35% of cases.27,28 Preexisting atherosclerotic plaque affecting all visceral vessels is the most common finding. Hypercoagulability syndromes can also predispose to acute visceral artery thrombosis. The affected segment of artery is usually its origin at the level of the aorta. Patients with acute arterial thrombosis frequently have preexisting symptoms of CMI. Schoots et al29 reviewed 45 observational studies encompassing 3692 patients with AMI and found that mortality from acute thrombosis of a mesenteric artery was 77.4%, compared with 54.1% for patients with acute arterial embolism to visceral vascular beds. This increased mortality is likely due to the involvement of larger segments of bowel because occlusions are typically within the first 2 cm of the SMA origin (Fig. 151-1B). This theory was corroborated in a Swedish study, in which 213 patients with acute thromboembolic occlusion of the SMA and intestinal infarction were examined post mortem.30 The cause of occlusion was embolic in 57.3% of patients and thrombotic in 41.3% (indeterminate in 1.4%). The extent of intestinal infarction was significantly greater in patients with SMA thrombosis compared with embolus. Acute extension of an aortic dissection can also serve as a mechanism for abrupt mesenteric vessel occlusion and thrombosis.
Nonocclusive Mesenteric Ischemia
Impaired intestinal perfusion in the absence of thromboembolic occlusion is termed nonocclusive mesenteric ischemia (NOMI) and makes up approximately 5% to 15% of cases.31 Visceral ischemia can occur from a low-flow state, which is exacerbated by the presence of any intestinal atherosclerotic disease. It is theorized that in such circumstances, in an effort to maintain cardiac and cerebral perfusion, excessive sympathetic output results in mesenteric vasospasm. NOMI most commonly occurs secondary to cardiac disease, particularly severe congestive heart failure, and in patients who have undergone cardiac surgery. Atrial fibrillation, commonly a cause of cardiac thrombi and visceral embolization, can also induce NOMI by reducing left ventricular function and causing low cardiac output. Other risk factors for NOMI include age, hypovolemia, systemic vasoconstrictors, vasoactive drugs (e.g., digoxin, α-adrenergic agents, β-receptor blocking agents, cocaine), aortic insufficiency, cardiopulmonary bypass, abdominal and cardiovascular surgery, abdominal compartment syndrome, and liver failure.25,32
In recent years, NOMI has been increasingly observed in patients on hemodialysis. Quiroga et al33 reported incidence rates more than 40 times greater among patients on hemodialysis compared with the general population. Using the Taiwan National Health Insurance Research Database, Li et al34 similarly reported a 44-fold higher risk of mesenteric ischemia in patients on hemodialysis or peritoneal dialysis compared with the general population. Older age and a heavier burden of cardiovascular risk factors undoubtedly contribute to these disparities. In addition, patients with end-stage renal disease are frequently taking erythropoietin, which elevates red cell mass and can lead to relative hyperviscosity. However, hypotension during dialysis has been implicated as the most important and immediate precipitating risk factor for development of NOMI.35 Therefore, special care should be made to avoid dialysis-related hypotension in patients at particularly high risk. High-risk patients include those who are older, are taking erythropoietin, have a longer history of dialysis, and are diabetics.33,35
Mesenteric Venous Thrombosis
MVT constitutes 5% to 15% of all cases of mesenteric ischemia.36,37 Involvement is usually limited to the superior mesenteric vein, but the inferior mesenteric, splenic, and portal veins can also be involved. MVT is classified as either primary (idiopathic) or secondary. Secondary MVT occurs when an underlying disease process is present; this type accounts for 90% of all patients with this disorder. Conditions associated with MVT can be categorized into three major categories: direct injury, local venous stasis or congestion, and thrombophilia. Causes of direct injury include abdominal surgery, trauma, and local inflammation, such as that seen with inflammatory bowel disease, pancreatitis, and diverticulitis.38,39 Splanchnic venous stasis may occur in the case of increased intra-abdominal pressure, obesity, and intraoperative manipulation of the mesenteric vessels. Inherited or acquired hypercoagulable diseases, including protein C and protein S deficiency, myeloproliferative disorders, antithrombin III deficiency, antiphospholipid antibody syndrome, and factor V Leiden mutation, are frequent causes of thrombophilia and thus also predispose to MVT.40
The extent of bowel ischemia depends largely on the degree of venous involvement and the presence of collateral vessels. The transition from normal to ischemic intestine is slower with MVT than with arterial occlusive disease.25 Edema and hemorrhage of the intestinal wall are frequently seen, followed by focal sloughing of the mucosa.25 The origin of thrombosis varies, depending on the etiologic process. When an intra-abdominal process is the cause, thrombosis begins in the larger mesenteric veins and progresses to involve the smaller venous arcades and arcuate channels.41 MVT caused by hypercoagulable conditions usually begins in the smaller mesenteric veins. Symptomatic acute MVT is associated with a 20% to 50% mortality rate.41 A Swedish population-based autopsy study showed that MVT-related death is more likely in patients with portal vein thrombosis, systemic venous thromboembolism, and obesity.42
Clinical Presentation
Acute Mesenteric Ischemia
The most common symptom of AMI associated with arterial thromboembolic disease is the sudden onset of abdominal pain. Because of a lack of collateral flow to the visceral organs, the presentation of AMI is more dramatic and severe, often with rapid clinical deterioration. Nausea, vomiting, diarrhea, emptying symptoms, and distention can also occur. Classically, the pain is out of proportion to the findings on physical examination. Initially, bowel sounds are hyperactive as the failure to relax the bowel smooth muscle leads to emptying symptoms. Bowel sounds are typically diminished in the later stages. Abdominal guarding and rebound tenderness are absent in the early stages of AMI; however, as intestinal ischemia and infarction progress, these signs become more pronounced. They are typically late findings, so their absence should not delay the diagnosis and treatment of AMI. Other late findings include fever, oliguria, dehydration, confusion, tachycardia, and shock.25 Metabolic abnormalities can include leukocytosis, metabolic acidosis, hyperamylasemia, elevated liver function values, and lactic acidemia.
Patients with NOMI or MVT typically present with a slower, more insidious clinical course. Frequently, patients with NOMI are critically ill, hospitalized, intubated patients who experience a sudden deterioration in their clinical condition. These patients are often administered intravenous pressors, worsening mesenteric vasoconstriction and thus decreasing splanchnic perfusion. In patients with MVT, fever, abdominal pain and distention, nausea and vomiting, and bloody stools are the most common findings. Dehydration and profound fluid shifts lead to bloody ascites and a hypovolemic state, causing further propagation of venous thrombosis.25 Although nonspecific, normal D-dimer levels may help rule out MVT.43
To develop better diagnostic criteria to reduce delays in diagnosis, Mitsuyoshi et al44 reviewed their 13-year experience treating 22 patients with NOMI. Multidetector row computed tomography (MDCT) was unavailable at the time the first 13 patients were treated, and 9 subsequently died of intestinal necrosis. These first 13 cases were used to devise four criteria for determining which patients warranted MDCT evaluation once this technology became available: (1) ileus or abdominal pain, (2) catecholamine requirement, (3) episode of hypotension, and (4) gradual rise in serum transaminase level. If three of the four criteria were present, patients received MDCT and underwent treatment with high-dose intravenous prostaglandin E1. Of the nine patients treated with this algorithm, only one died.
Chronic Mesenteric Ischemia
Postprandial abdominal pain and progressive weight loss are the most common symptoms in patients with CMI. Pain is often described as dull and crampy and located in the midepigastric region. Pain often occurs 15 to 45 minutes after a meal, and the severity varies according to the size and type of meal. Patients typically develop “food fear” and decrease their oral intake in anticipation of severe pain after meals. Consequently, weight loss is a common finding and in fact, when present, can help distinguish CMI from other functional bowel disorders when the diagnosis is in question.45 Changes in bowel habits, nausea, and vomiting are less common findings. CMI is seen more frequently in elderly women, who represent 70% of patients. The variable nature of symptoms often makes the diagnosis confusing and can result in delayed treatment. The traditional risk factors for atherosclerosis are usually present. A heavy smoking history is frequently obtained, and the majority of patients also have a history of symptomatic manifestations in other vascular beds, most commonly cerebrovascular, coronary, and peripheral arteries.46
Physical examination findings are usually nonspecific. Patients are commonly undernourished and cachectic (Fig. 151-2). An abdominal bruit can sometimes be auscultated but is not always present. Bowel sounds are frequently hyperactive. Guarding and rebound tenderness are usually absent. Low prealbumin and albumin levels are often seen owing to the patient’s chronic malnourished state.
Diagnostic Evaluation
Noninvasive Evaluation
DUS is a useful tool for the early, noninvasive diagnosis of visceral ischemic syndromes. Color Doppler scanning can be used to assess the flow velocities and resistance index in the splanchnic arteries and their arterial beds as well as to evaluate end-organ vascularity. The intestinal wall can also be assessed with a high degree of accuracy by high-resolution transabdominal ultrasound.47 Transmural hemorrhage, inflammation, and necrotic thickening in the bowel wall can be imaged sonographically. Asymmetrical wall thickening with associated ileus as well as ascites and free peritoneal air can be seen in patients with AMI.48 DUS combined with expiratory maneuvers is also an excellent screening examination for median arcuate ligament syndrome.
Moneta et al49 performed blinded DUS studies in 100 patients who previously underwent arteriography of the celiac trunk and SMA. They hypothesized that lack of flow or a peak systolic velocity (PSV) in the SMA of greater than 275 cm/s, or no flow or a PSV of greater than 200 cm/s in the celiac trunk, was a reliable indicator of 70% or greater angiographic stenosis.50 With use of these criteria, duplex sensitivity for detection of lesions in the SMA and celiac artery was 92% and 87%, respectively. Overall accuracy for detection of a 70% lesion in the SMA and celiac artery was 96% and 82%, respectively. The IMA is not generally assessed because it is generally difficult to visualize and is of lower clinical importance. However, patency of the IMA can usually be determined.
Limitations of transabdominal duplex scanning include the wide variation in examination quality, which is operator dependent. Patient-related factors, such as obesity, excessive intraluminal bowel gas, variation in local anatomy, and effects of respiration, can affect image quality.51 Care must be taken to clearly define the origin of each vessel to avoid inaccurate measurements. DUS is generally not recommended in the workup of AMI.
DUS is also the primary imaging modality used for surveillance after both bypass and stenting. However, no specific criteria exist for determining restenosis. Baker et al52 evaluated the accuracy and utility of DUS for detection of in-stent stenosis in 23 patients who underwent successful (<20% residual stenosis on completion angiography) mesenteric stenting for CMI (20 SMA alone, 3 both SMA and celiac artery). Preprocedure DUS was performed in 13 patients with a mean PSV of 464 cm/s. Initial surveillance DUS was performed in 21 patients at a mean of 0.9 month after revascularization. Mean PSV at this time was 335 cm/s, and for 12 of these patients, the first postoperative PSV in successfully stented vessels was higher than the 275 cm/s threshold used to diagnose high-grade native SMA stenosis. In addition, there was no correlation between surveillance PSV and the degree of angiographic stenosis seen at the time of re-intervention.
In their review of 107 patients who underwent endovascular therapy for CMI, Schoch et al53 similarly reported that although 83% of patients had recurrent stenosis on surveillance DUS, 53% of patients remained asymptomatic and required no further intervention. These findings therefore mandate obtaining an early baseline DUS against which future surveillance scans can be compared. They also stress the importance of considering the clinical context and symptom recurrence in making the decision to re-intervene.
The predictive value of DUS for detection of disease recurrence after mesenteric bypass has also been studied. Liem et al54 evaluated 167 duplex examinations used in the surveillance of 43 bypass grafts in 38 patients. On comparison of antegrade and retrograde bypass configurations, differences in mean PSV were demonstrated only at the inflow artery. There were no differences in PSV at the proximal or distal anastomosis, in the midgraft, or in the outflow artery. Between the first postoperative DUS examination and the latest follow-up scan (on average 38 months), PSV measurements did not change significantly. Graft failure occurred in two patients, both of whom had no findings to suggest impending occlusion on the DUS examinations immediately preceding failure. No predictors of graft thrombosis were identified on multivariable analysis.
Computed tomography (CT) is an accurate, noninvasive imaging modality for diagnosis of mesenteric ischemia. Modern MDCT enables imaging with excellent spatial and temporal resolution. A meta-analysis of six studies published between 1996 and 2009 on the diagnostic accuracy of MDCT in AMI showed a pooled sensitivity of 93% and specificity of 96%.55 Advantages over conventional angiography include the relative ease and speed of performance; the rapid infusion of contrast agent through peripheral intravenous lines; and the ability to simultaneously image the mesenteric arteries, veins, and visceral organs. CTA is also useful for evaluating patency of previously placed grafts and stents. Common radiographic findings in the bowel wall related to AMI include increased thickening, dilatation, and attenuation, which can be easily detected with CT. Pneumatosis intestinalis, portal venous air, mesenteric edema, and ascites can also be detected. During the arterial phase of contrast infusion, the mesenteric vessels can be evaluated for thrombosis, embolus, dissection, and aneurysm. Venous engorgement and subtle intestinal findings may be identified in cases of mesenteric venous occlusion. A “target sign” may be seen in the superior mesenteric vein, with thrombus in the center of the lumen and surrounding contrast-enhanced blood flow peripherally. The portal venous phase is more accurate for diagnosis of MVT, and images during the venous phase may be acquired if the diagnosis is still in question or for surveillance of clot burden or extension in patients with MVT. In many centers, a biphasic scan, which includes a delayed venous phase in addition to an arterial phase, has been used in the diagnosis of AMI because it enables detection not only of arterial occlusion but also of MVT and improves the ability to detect changes in the bowel wall as well.
Disadvantages of CT include radiation, risk of contrast nephropathy, and hypersensitivity reactions to iodinated contrast agents. Inaccurate timing of contrast infusion during the arterial phase may provide indeterminate images and delay diagnosis. Because calcification at the vessel origins enhances in a similar fashion to intravenous contrast material, it is possible to underestimate the degree of stenosis. Therefore, the non–contrast-enhanced images should be reviewed. In addition, with careful adjustment of the window and level, calcification can usually be distinguished from intravascular contrast material. Last, CT serves strictly as a diagnostic modality; treatment must be performed through a separate angiographic procedure or laparotomy.
MRA is useful for diagnosis of mesenteric occlusive disease. Although MRA takes significantly longer to perform than CTA, it avoids the radiation exposure associated with CTA. Patients with hypersensitivity to iodinated contrast agents may also benefit from MRA. However, CTA has superior spatial resolution and allows better visualization of the IMA, peripheral splanchnic vessels, calcified plaque, and previously placed stents, giving it a distinct advantage.26 For these same reasons, CTA is also preferred to MRA for surveillance after revascularization. Our standard workup includes DUS to screen those thought to have CMI. CTA is used routinely before intervention for AMI, CMI, and MVT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree