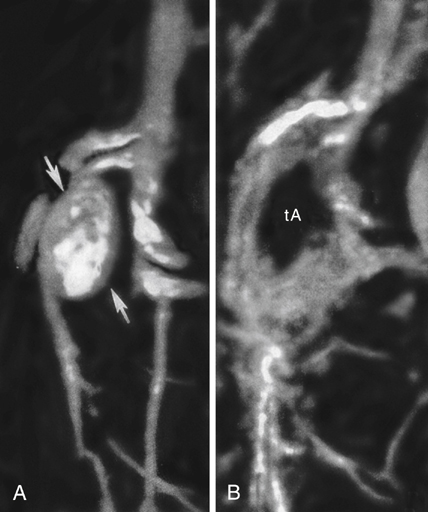
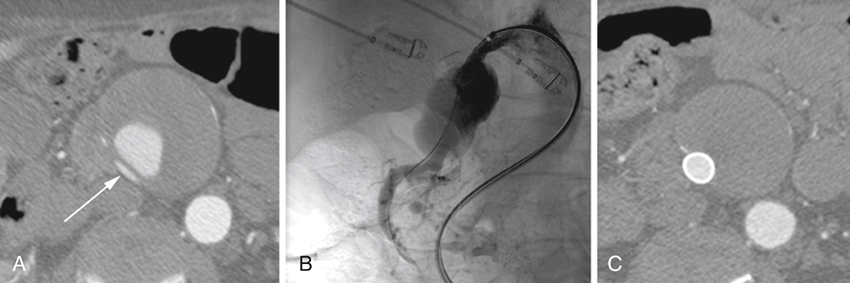
Aneurysms of the proximal superior mesenteric artery (SMA) are the third most common reported splanchnic artery aneurysm, accounting for 5.5% of these lesions. They are categorized as being true aneurysms or dissecting aneurysms (Figures 1 and 2). True aneurysms affect men nearly twice as often as women, and nearly 90% of dissecting aneurysms occur in men. Most SMA aneurysms are associated with an underlying medial degenerative process. Some are a result of a systemic arteriopathy such as occurs in Ehlers–Danlos syndrome. Infectious SMA aneurysms were common in the past, but they are encountered infrequently in current practice. When mycotic SMA aneurysms are encountered they are usually secondary to organisms within emboli arising from bacterial endocarditis, mostly nonhemolytic streptococci. SMA aneurysms may also be related to periarterial inflammation and to trauma, manifesting as pseudoaneurysms. These pseudoaneurysms carry much greater risks to the patient than either true or dissecting SMA aneurysms. Arteriosclerosis, when present, is considered a secondary event rather than an etiologic process.
The majority of true SMA aneurysms are usually recognized during imaging studies for other diseases. Although ultrasonography or roentgenography undertaken for evaluation of musculoskeletal back or vague abdominal discomfort can provide evidence of an aneurysm, more definitive imaging is required to establish a diagnosis, including magnetic resonance arteriography (MRA), computed tomography arteriography (CTA), or conventional catheter-based arteriography. Conventional arteriography is particularly favored if an intervention is being considered. In such cases it is important to define the pattern of flow in the collateral vessels originating from both the celiac and inferior mesenteric circulations.
Most true SMA aneurysms today are asymptomatic. Symptomatic aneurysms can accompany acute expansion, mural dissection, or frank rupture. The potential for rupture remains ill defined, although rupture affected a little more than a third of cases reported from the Mayo Clinic. This rate of rupture is not consistent with most other reports, and a rupture rate near 10% is likely closer to the true incidence of rupture. Mortality approaches 50% with rupture.
Spontaneous aneurysmal SMA dissections unassociated with an aortic dissection are rare. They usually originate a few centimeters from the SMA’s aortic origin and rarely extend for more than 4 or 5 cm. A majority of patients with this type of aneurysm come to the hospital with severe epigastric and occasional back discomfort, similar to that occurring with an episode of acute pancreatitis, accompanied with nausea and vomiting. The unique location of SMA dissecting aneurysms near the origins of the inferior pancreaticoduodenal and middle colic arteries place the distal SMA circulation at risk if these vessels become occluded with aneurysmal thrombosis. In this setting there is a resultant loss of the usual collateral networks from the adjacent celiac and inferior mesenteric arterial circulations, and profound intestinal ischemia with small bowel infarction can follow. In an occasional patient, the latter may be responsible for chronic intestinal ischemia with postprandial pain and eventual weight loss associated with avoidance of eating, suggesting abdominal angina.
Long-term studies on the natural history of asymptomatic true or dissecting SMA aneurysms unassociated with an underlying arteriopathy are nonexistent and thus rigid criteria for their therapy are not available. Nevertheless, we consider that intervention is warranted in asymptomatic true SMA aneurysms greater than 2 cm in diameter, those manifesting documented expansion, and all symptomatic aneurysms. Controversy surrounds dissecting SMA aneurysms, and many of these lesions that are asymptomatic can regress as blood within the mural dissection is reabsorbed and be of no major risk to the patient. Most patients in this setting with a partial occlusion of the SMA lumen by the mural hematoma may be treated by anticoagulation alone. However, nearly 40% of patients treated conservatively in this manner eventually require an open or endovascular procedure, and they must be followed closely when they are managed conservatively. Dissecting SMA aneurysms that are symptomatic or exhibit expansion are best treated initially with an open or endovascular repair.
A conventional open operation with an SMA aneurysmectomy and vascular reconstruction of the SMA may be performed in good-risk patients in whom anatomic features of their SMA circulation preclude an endovascular intervention. In these cases, the reconstruction can necessitate an aortomesenteric bypass or aortic reimplantation of the normal SMA beyond the aneurysm. The latter requires mobilization of the SMA at the base of the mesentery, with aortic implantation below the left renal vein. An occasional patient having a small eccentric aneurysm may be treated with a closed aneurysmorrhaphy. Somewhat surprisingly, a modest number of SMA aneurysms are simply ligated, with no attempt to reconstruct the SMA itself (Figure 1). This may be quite appropriate in instances where the SMA aneurysm has thrombosed earlier and the patient has remained asymptomatic. The fact is that ligation has been the most common reported open surgical means of managing these lesions. Certainly, if ligation is considered and temporary SMA clamping intraoperatively causes bowel ischemia, then a formal intestinal revascularization becomes mandatory.
Endovascular stent-graft placement has appeal for selected SMA aneurysms, especially those associated with chronic dissections (see Figure 2). Although late complications of thrombosis and infection can accompany endovascular stent-graft therapy, the early morbidity and mortality are much less than occurs with an open surgical procedure. Obliteration of SMA aneurysms by coils or direct thrombin injection may be preferred in carefully selected high-risk patients with discrete aneurysm necks. These obliteration interventions require a high degree of skill because inadvertent SMA embolization of thrombogenic material can lead to devastating bowel ischemia.