Chapter 32 Melanoma and Cutaneous Malignancies
Cutaneous Melanoma
In 1787, Hunter published one of the first accounts of a patient with melanoma. Laennec, who found metastatic melanoma deposits in distant viscera, described melanoma as cancer noire, or the black cancer, in 1806. He subsequently named the disease melanosis in 1812.1
Epidemiology and Causes
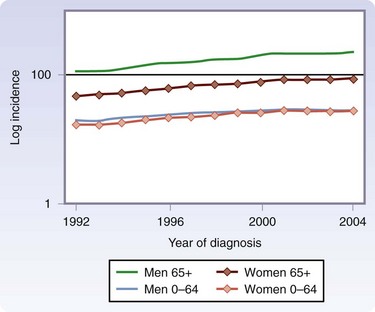
Melanoma is now the fifth most common cancer in men and the sixth most common cancer in women in the United States. Although melanoma accounts for less than 5% of skin cancer cases, it causes the vast majority of skin cancer deaths.2 The incidence is greatest in Australia and lowest in Japan among developed countries (Fig. 32-1).3 The American Cancer Society has estimated that approximately 70,230 new melanomas were diagnosed in the United States in 2010. The incidence of melanoma has risen sharply in the past few decades (Fig. 32-2).4 During the 1970s, its incidence rose at a rate of about 6%/year. Fortunately, the increase slowed to less than 3%/year during the 1980s and 1990s and, since 2000, it has been relatively stable. It is estimated that there were 8790 deaths from melanoma in 2010 in the United States. The death rate has been stable since 1990, although it continues to rise gradually for men aged 65 years or older (Fig. 32-3).
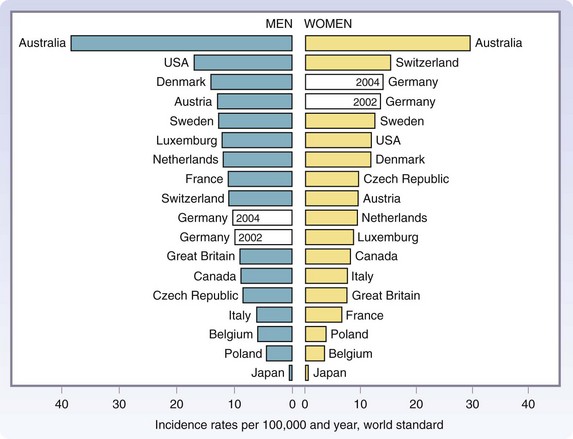
FIGURE 32-1 Age-standardized (world standard population) incidence rates from 17 countries worldwide for the year 2002.
(From Garbe C, Ulrike L: Melanoma epidemiology and trends. Clin Dermatol 27:3–9, 2009.)
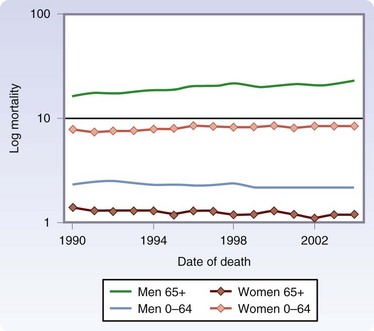
FIGURE 32-3 Age-adjusted mortality rates from melanoma/100,000 according to age and gender, 1990-2004.
(From Linos E, Swetter SM, Cockburn MG, et al: Increasing burden of melanoma in the United States. J Invest Dermatol 129:1666–1674, 2009.)
Cutaneous melanoma is predominantly a disease of whites. The degree of pigmentation in the skin is a relative protective factor against cutaneous melanoma. Risk factors for melanoma include high-risk skin type (e.g., those with blue eyes, blond or red hair, fair complexion), reaction to sun exposure (e.g., freckling, inability to tan, propensity for sunburn), a history of severe blistering sunburns, intense intermittent sun exposure, upper socioeconomic status, family history of melanoma, large number of nevi, giant congenital nevi, presence of dysplastic nevi, immunosuppression, history of prior melanoma or other skin cancers, and xeroderma pigmentosum. The average annual age-adjusted melanoma incidence/100,000 persons is 18.4 for whites compared with 2.3 for Hispanics, 1.6 for Asians, 1.0 for Native Americans, and 0.8 for African Americans. Melanoma is slightly more common in males than in females.5,6
The causes of melanoma are not completely defined, but it is clear that exposure to ultraviolet (UV) radiation is a key causative factor. UV wavelengths are classified as UVA or UVB; UVA has the longer wavelength, 320 to 400 n, and UVB ranges from 290 to 320 nm. UVA, the predominant UV wavelength in tanning beds and lights, penetrates more deeply into the skin than UVB. Although UVA radiation has long been known to play a major role in skin aging and wrinkling, growing evidence has implicated UVA radiation in the cause of nonmelanoma skin cancers as well as melanomas. In melanoma centers around the country, teenagers and young adults are often found with melanoma, predominantly young women who almost universally have been tanning bed users. UV radiation exposure from the sun is a major risk factor, especially among those with fair skin who are more susceptible to sunburns. As compared with nonmelanoma skin cancers, which appear to be related more to chronic repeated sun exposure, melanoma might be related more to intermittent sun or UV radiation exposure. UVB damages the skin’s more superficial epidermal layers and is the chief cause of sunburn; it has long been implicated in the development of melanoma.7
Precursor Lesions
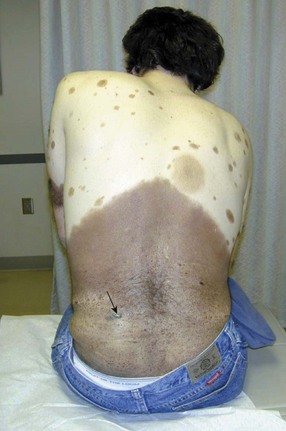
The risk of those with congenital nevi is proportional to the size and number of nevi. Small congenital nevi represent a low risk and are therefore observed unless they change in appearance. Giant congenital nevi (>20 cm in diameter) are rare (1 in 20,000 newborns), but carry an increased lifetime risk for the development of melanoma of up to 10%. Complete excision should be considered, when possible (Fig. 32-4). At a minimum, these patients should undergo regular dermatologic evaluation.8
Spitz nevus (juvenile melanoma, spindle cell melanoma, epithelioid cell melanoma) is a rapidly growing, pink or brown, benign skin lesion arising most often in children, although adult skin lesions may also have spitzoid features. Spitz nevus may be difficult to distinguish histologically from melanoma. Consultation with an expert dermatopathologist is recommended; however, even the best pathologists may have difficulty in determining the malignant potential of Spitzoid tumors. Although complete excision with negative margins is adequate for an unequivocal Spitz nevus, often the diagnosis is not so clear-cut. If there is any concern that the lesion may be melanoma, WLE with margins appropriate for melanoma is performed. Sentinel lymph node (SLN) biopsy has been proposed as a mechanism to clarify the malignant potential in indeterminate cases, although this is controversial.9
Familial Melanoma
A hereditary basis has been established for a minority of patients with melanoma. Variously termed dysplastic nevus syndrome, familial atypical multiple mole-melanoma (FAMMM) syndrome, and B-K mole syndrome, among others, these syndromes include patients with melanoma in one or more first- or second-degree relatives and large numbers of melanocytic nevi (often 50 to 100 or more), some of which are clinically and histologically atypical or dysplastic. There may also be a family history of other malignancies, especially pancreatic cancer. These patients require detailed dermatologic evaluation several times annually, with periodic biopsies of the most suspicious lesions. Mutations in the gene CDKN2A in the 9p21 region have been demonstrated in familial melanoma kindreds. The CDKN2A gene is complex and codes for p16 and p14ARF, which both function to suppress cellular proliferation. Mutations in cyclin-dependent kinase 4 (CDK4) and cyclin-dependent kinase inhibitor 2A (CDKN2A) have also been implicated.5,10
Prevention
Much excessive UV radiation exposure, in the form sunbathing and tanning bed use, is intentional and completely preventable. Recommendations for reducing UV radiation exposure include avoiding these activities, use of protective clothing, and use of sunscreens. Although most experts believe that the use of sunscreens will reduce the risk of melanoma, this topic is controversial, because some have suggested that sunscreens may provide a false sense of security and allow persons at risk to experience more prolonged sun exposure. However, a meta-analysis of 18 studies11 has found no evidence that use of sunscreens increased the incidence of melanoma.5 Regular dermatologic evaluation of patients with suspicious pigmented skin lesions is prudent.
Diagnosis
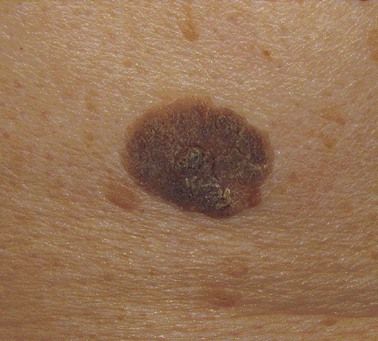
Melanoma presents most commonly as an irregular pigmented skin lesion that has grown or changed over time. Melanomas most commonly arise de novo, but may also arise within a congenital or acquired nevus. The distinction between a benign nevus and an early melanoma can be difficult, even for experienced clinicians. Benign pigmented lesions are so prevalent that it is challenging to detect an early melanoma among many benign lesions. The most common pigmented skin lesions are seborrheic keratoses, known as the barnacles of life because of the propensity for patients to acquire them with increasing age (Fig. 32-5). These are typically scaly, waxy, raised lesions with a stuck-on appearance that seem as if they could be scraped off with a fingernail; the characteristic appearance usually is completely diagnostic and these lesions do not need to be removed. However, even the most experienced dermatologists have been fooled by what appeared to be an irritated seborrheic keratosis and turned out to be melanoma.
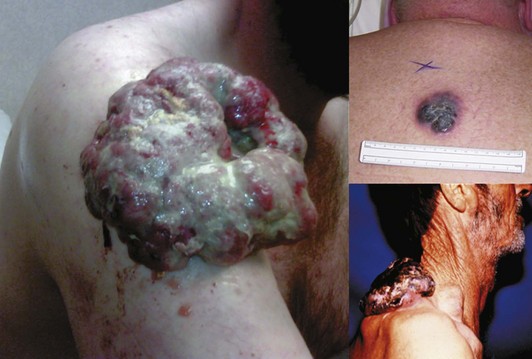
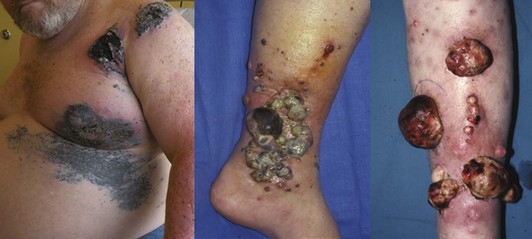
The ABCDEs of melanoma are used to guide the decision about performing a biopsy—asymmetrical irregular borders, variable shades of color, diameter greater than 6 mm, and evolution, or change over time. However, many melanomas do not follow these rules. Amelanotic melanomas are not pigmented and may present as a raised pink or flesh-colored skin lesion. A high index of clinical suspicion is needed, and particular attention should be paid to any history of change in a lesion. If a patient presents with a skin lesion that has changed in size, color, or shape, and/or is itching or bleeding, a biopsy should be performed. To tell a patient that it will just be observed means that it will be ignored. There should be a low threshold for performance of biopsy. Fortunately, locally advanced melanomas are now infrequently encountered, given the increased awareness of this disease (Fig. 32-6).
Biopsy
For larger lesions, it may be appropriate first to get a tissue diagnosis prior to performing complete excision; this is accomplished by a full-thickness incisional biopsy. The simplest way to perform an incisional biopsy is by use of a punch biopsy. A punch biopsy is performed using a disposable instrument that removes a cylinder of skin and subcutaneous tissue (2 to 8 mm in diameter) by simply twisting the instrument into the anesthetized skin, followed by closure with one or two simple sutures (Fig. 32-7). Punch biopsies of at least 4 mm should be performed, because a 2-mm punch often does not provide adequate tissue for pathologic evaluation. The punch biopsy should be performed through the thickest area(s) of the lesion, and multiple punch biopsies can be performed to sample larger lesions. Shave biopsies are frequently performed by dermatologists and are appropriate for many nonpigmented skin lesions. This is a good way to diagnose squamous cell and basal cell carcinoma. A shave biopsy is performed by elevating the skin lesion with forceps or inserting a small needle beneath the lesion, followed by shaving the lesion with a razor blade or scalpel. Hemostasis is achieved using topical agents such as ammonium chloride or by electrocautery. The patient then treats the area with topical antibiotic ointment and it is allowed to heal by secondary intention. Because a shave biopsy is quick and simple to perform and does not require sutures, it is a popular method of biopsy. However, shave biopsy should generally be discouraged for pigmented lesions, because if a melanoma is diagnosed, a shave biopsy may transect directly through the melanoma and not allow an accurate assessment of tumor thickness as the base of the lesion is cauterized. Therefore, shave biopsy should not be used when melanoma is suspected. To circumvent this problem, dermatologists often perform deep shave or saucerization biopsies, which completely remove the lesion down to subcutaneous fat if there is any concern for melanoma. In the hands of experienced clinicians, this can be an effective biopsy technique. All pigmented lesions should be sent for pathologic evaluation.12 Ablation of pigmented skin lesions using cryotherapy, cautery, or lasers should be specifically discouraged; there are many examples of disastrous delays in diagnosis as a result of such practices.
Pathology
Histologically, invasive cutaneous melanoma is divided into four major types based on growth pattern and location. These forms are lentigo maligna melanoma, superficial spreading melanoma, acral lentiginous melanoma, and nodular melanoma. Melanomas arise as proliferations of melanocytes in the basal layer of the skin. As they multiply, these cells expand radially in the epidermis and superficial dermal layer, termed the radial growth phase. With time, the growth begins in a vertical direction as the skin lesion may become palpable, the so-called vertical growth phase. Nodular melanomas are an exception to this pattern, wherein the vertical growth phase is present early in tumor development. The vertical growth phase allows invasion into the deeper layers of the skin, where the tumor may achieve metastatic potential by invasion of blood and lymphatic vessels.13
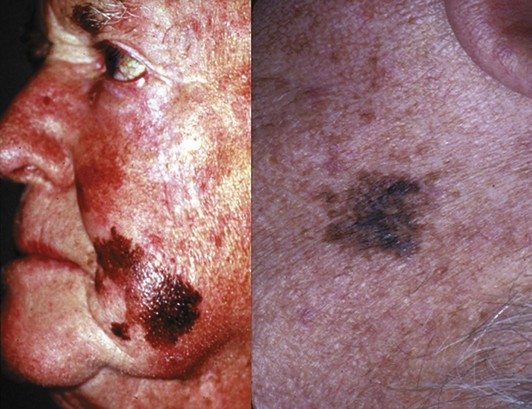
The histologic subtype of melanoma is not, in general, a major factor in prognosis; tumor thickness, ulceration, and other factors determine the prognosis. However, some histologic subtypes are more likely to be detected at a more advanced stage. Lentigo maligna melanoma occurs most commonly on the face of older individuals with sun-damaged skin and presents as a flat, dark, variably pigmented lesion, with irregular borders and a history of slow development (Fig. 32-8). Lentigo maligna melanomas may become large prior to diagnosis, because the slow progression may escape the patient’s notice. Overall, the prognosis of lentigo maligna melanomas is better than for the other histopathologic types because of the often superficial nature of these tumors. However, lentigo maligna melanomas can pose challenging management problems because of their propensity to develop in cosmetically challenging areas (e.g., face), and the fact that the histologic extent of the lesion may extend well beyond the clinically apparent borders of the pigmented lesion. Thus, achieving negative margins may be challenging. Before embarking on complex tissue flaps for closure, it is prudent to ensure negative margins. This may necessitate delaying the closure until the final pathology report indicates negative margins of excision.
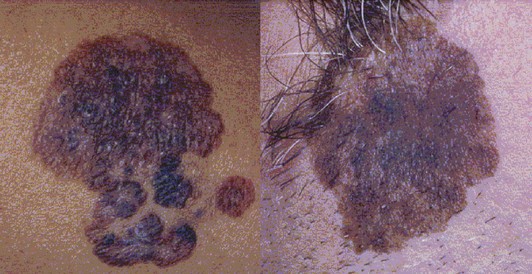
The most common histologic type is superficial spreading melanoma (Fig. 32-9). It is not necessarily associated with sun-exposed skin. As the name suggests, superficial spreading melanoma initially appears as a flat pigmented lesion growing in the radial dimension. If allowed to progress, these melanomas develop a vertical growth phase and invade more deeply into the skin.
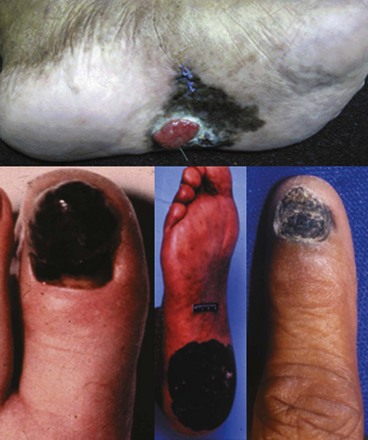
Acral lentiginous melanoma (ALM) is classified by its anatomic site of origin. These tumors develop in the subungual areas, beneath the fingernails and toenails, and on the palms of the hand and soles of the feet (Fig. 32-10). This is the most common type of melanoma in black patients. The histologic appearance of ALMs is similar to melanomas arising on the mucous membranes. The diagnosis is often made at an advanced stage, which accounts for the poor prognosis of these tumors in general. Subungual acral lentiginous melanomas are often mistaken for a subungual hematomas, leading to a delay in diagnosis. A key feature distinguishing a subungual melanoma from a subungual hematoma is that for subungual hematoma, the pigment should migrate distally with growth of the nail. Biopsy of subungual melanomas can be accomplished by performing a digital block with local anesthesia and removing the nail or performing a punch biopsy through the nail itself.
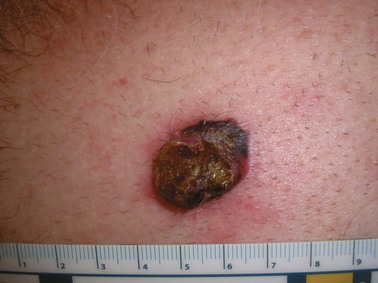
Nodular melanomas are raised papular lesions that develop a vertical growth pattern early in their course (Fig. 32-11). These melanomas often have a poor prognosis because of greater average tumor thickness and frequent ulceration.
Prognostic Factors
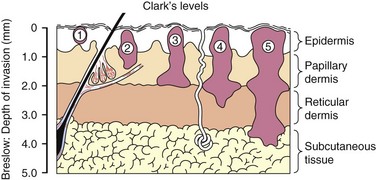
In 1969, Dr. Wallace Clark described a classification of melanoma based on the level of invasion into the anatomic layers of the skin. Henceforth known as Clark’s level of invasion, this classification scheme correlated with survival (Fig. 32-12).14 Clark level I tumors represent melanoma in situ and are limited to the epidermis; therefore, these lesions do not have metastatic potential. Clark level II melanomas extend into the papillary dermis, level III fill the papillary dermis, level IV to the reticular dermis, and level V extend to the subcutaneous fat. In 1970, Dr. Alexander Breslow described a simpler system based on measurement of the vertical thickness of the melanoma in millimeters, now known as Breslow’s thickness.15 As the thickness of the melanoma increases, the prognosis worsens.
The status of the regional lymph nodes is the single most important prognostic factor predicting survival. Metastasis to the regional lymph nodes increases the chances of mortality from melanoma substantially. The other major prognostic factors, in order of importance, are Breslow thickness, ulceration, age, anatomic location of the primary tumor, and gender. Mitotic rate is a more recently validated prognostic factor that may also be important to consider, especially among patients with thin melanomas. Ulceration has emerged as a robust predictor of prognosis. Ulceration is defined pathologically as the absence of an intact epithelium overlying the melanoma. Patients with ulcerated melanomas have a worse prognosis than those with nonulcerated melanomas, even among patients with regional nodal metastasis. Why patients with ulcerated melanomas have a worse prognosis is unclear, but ulceration appears to be a phenotypic marker for worse tumor biology and greater propensity for invasion and metastasis. Older patients have a greater risk of melanoma mortality than younger patients, despite the fact that younger patients are more likely to have nodal metastasis. Patients with axial (trunk, head, and neck) melanomas have a worse prognosis than those with extremity tumors. Regression has not been shown to be an important factor predicting nodal metastasis or survival. Women have a better prognosis than men, for reasons that are unclear.16
Staging
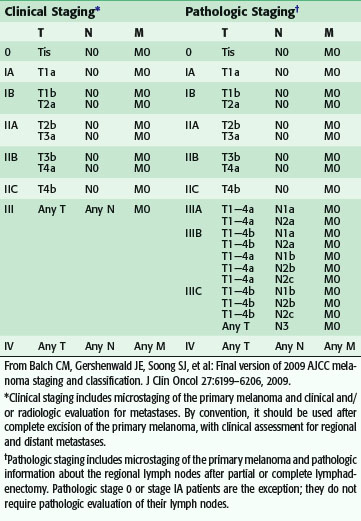
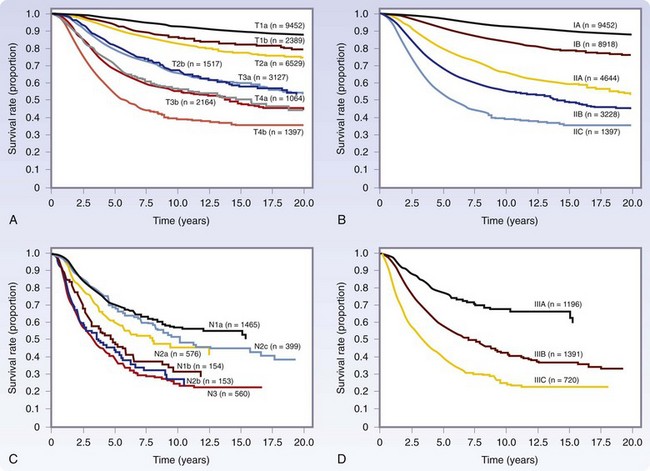
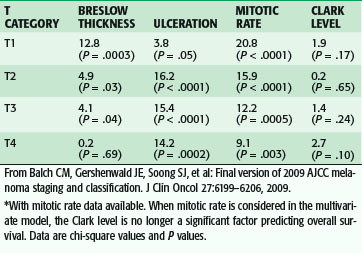
Staging for cutaneous melanoma uses the tumor-node-metastasis (TNM) system of classification as defined by the AJCC (Tables 32-1 and 32-2). The 2009 version (seventh) of the staging system is based on analysis of a database of over 30,000 patients.17 The important prognostic factors in the staging system include Breslow thickness, ulceration, nodal status, and presence of other manifestations of lymphatic spread (e.g., satellite lesions, in-transit disease), as well as the presence of distant metastatic disease. The principal change from the previous (sixth) version is that a mitotic rate of one mitosis/mm2 or more is now used, instead of Clark level, to discriminate T1a from T1b tumors based on the finding that the mitotic rate is a more powerful predictor of prognosis (Table 32-3). This system provides excellent discrimination of survival among various stages of disease (Fig. 32-13). Based on the work of Balch, collaborators from the AJCC staging committee developed an online tool for the assessment of prognosis based on individual patient characteristics (www.melanomaprognosis.org).
Table 32-1 TNM Staging Categories for Cutaneous Melanoma
| T CLASSIFICATION | THICKNESS | ULCERATION STATUS AND MITOSES |
|---|---|---|
| T1 | ≤1.0 mm | |
| T2 | 1.01-2.0 mm | |
| T3 | 2.01-4.0 mm | |
| T4 | >4.0 mm | |
| N CLASSIFICATION | NO. OF METASTATIC NODES | NODAL METASTATIC MASS |
| N1 | One node | |
| N2 | Two or three nodes | |
| N3 | Four or more metastatic nodes, or matted nodes, or in-transit metastases-satellite(s) with metastatic node(s) | |
| M CLASSIFICATION | SITE | SERUM LDH LEVEL |
| M1a | Distant skin, subcutaneous, or nodal mets | Normal |
| M1b | Lung metastases | Normal |
| M1c | All other visceral metastases | Normal |
| Any distant metastasis | Elevated |
* Micrometastases are diagnosed after sentinel lymph node biopsy and completion lymphadenectomy (if performed).
† Macrometastases are defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension.
From Balch CM, Gershenwald JE, Soong SJ, et al: Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206, 2009.
Table 32-3 Multivariate Cox Regression Analysis of Pathologic Factors by T Category for Stages I and II Melanoma*
Initial Evaluation
Most melanoma patients who seek surgical consultation will have already been diagnosed with melanoma. The first and most important evaluation of a patient diagnosed with melanoma is a thorough history and physical examination. The history should elicit factors related to the primary melanoma (e.g., duration, change over time, symptoms such as itching and bleeding) and other factors, such as sun exposure, tanning bed use, immunosuppression, prior history of cancer, and family history. Melanoma may be localized or may metastasize to regional or distant sites. Regional metastasis refers to the lymphatic spread of tumor to the regional lymph nodes, those in the immediate lymphatic drainage pathway from the site of the primary tumor. In-transit melanoma is a form of regional lymphatic metastasis in which the tumor spreads within the draining lymphatic channels and becomes evident as cutaneous or subcutaneous nodules between the site of the primary tumor and regional lymph nodes (Fig. 32-14). Distant metastasis refers to the hematogenous spread of melanoma to distant organs. Although uncommon at the time of initial diagnosis, it is also important to elicit symptoms of metastatic disease, such as any masses, neurologic symptoms or headaches, anorexia, weight loss, bone pain, or respiratory symptoms. A detailed physical examination should specifically include a complete skin examination, with inspection and palpation of the skin to detect any other suspicious skin lesions, including detection of in-transit disease. Palpation of the cervical, axillary, and inguinal lymph nodes should be performed, and palpation of the epitrochlear or popliteal nodes, as appropriate for distal upper or lower extremity melanomas, respectively. When present, all symptoms and signs of metastasis require further radiologic evaluation.
The National Comprehensive Cancer Network (NCCN), a consortium of oncologists from National Cancer Institute–designated cancer centers, provides consensus-based guidelines for cancer treatment. These guidelines for the management of primary melanoma are available online (www.nccn.org).
Extent of Disease Evaluation
There is controversy regarding the extent of imaging evaluation appropriate for patients with melanoma. For most stages I and II patients, additional imaging studies are unnecessary, although imaging evaluation may be appropriate in patients with thick primary tumors (stage IIC). The role of imaging tests for stage III patients with disease detected by sentinel node biopsy is controversial. Because the probability of detecting true-positive findings on studies such as positron emission tomography (PET) and computed tomography (CT) scanning is extraordinarily low in patients with microscopic nodal metastasis; there is a real danger of false-positive results, but these imaging tests should be ordered and interpreted with caution. Patients with clinically detectable nodal metastasis should undergo imaging studies to evaluate for the presence of distant metastatic disease, because the distinction between stages III and IV disease is important in determining the appropriate treatment options. Furthermore, for patients with stage IV melanoma, imaging evaluation is necessary to determine the extent of disease and whether resection is appropriate. In such cases, PET, CT, and magnetic resonance imaging (MRI) of the brain are generally recommended.18
Treatment
Surgical Management
Wide Local Excision
The operation to resect the primary melanoma is known as WLE. The appropriate margins for excision have long been a topic of controversy. In 1857, Norris suggested WLE for a primary melanoma to prevent local recurrence and advocated a 5-cm margin, a recommendation that would be followed for over a century.19 Until the 1960s, all melanomas were considered to be aggressive tumors and were often treated with very wide margin excision. Current guidelines for WLE are given in Table 32-4.
Table 32-4 Recommended Margins of Wide Local Excision (WLE)
| THICKNESS (mm) | WLE MARGIN (cm)* |
|---|---|
| In situ | 0.5 |
| <1 | 1 |
| 1-2 | 1-2† |
| >2-4 | 2 |
| >4 | 2‡ |
* Smaller margins may be justified in specific cases to achieve better functional or cosmetic outcome.
† A 1-cm margin may be associated with a slightly greater risk of local recurrence in this Breslow thickness category.
‡ There is no evidence that margins >2 cm are beneficial; however, larger margins may be considered for advanced melanomas when local recurrence risk is high.
From Balch CM, Gershenwald JE, Soong SJ, et al: Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206, 2009.
Melanoma in situ is generally adequately treated by a 0.5-cm margin excision; this is not based on any randomized data, but on clinical experience with this disease entity. However, given that there is significant interobserver variability among pathologists in its diagnosis,20 and that early invasive melanoma may be diagnosed after WLE if the entire lesion was not removed during the initial biopsy, it is often prudent to perform a 1-cm WLE for melanoma in situ that occurs in anatomic areas that easily allow a 1-cm margin with primary closure (e.g., the trunk).
Several randomized studies have been performed to assess the margin width for intermediate-thickness melanomas.21,22 The first trial, conducted by the World Health Organization, randomized 612 patients with melanomas 2-mm thick or less to WLE using a 1- or 3-cm margin. Local recurrence as a site of first recurrence was observed in four patients, all with melanomas larger than 1 to 2 mm thick who were in the 1-cm margin group; however, this did not significantly affect overall survival. The Intergroup Melanoma Trial randomized 462 patients with melanomas of the trunk or proximal extremities between 1-mm and 4-mm Breslow thickness to receive WLE with a 2-cm or 4-cm margin. After a median follow-up of 10 years, the incidence of local recurrence was the same for patients undergoing 2-cm or 4-cm margin excision (2.1% versus 2.6%, respectively); there was no significant difference in overall survival. Factors significantly associated with local recurrence were ulceration, thickness, and anatomic location of the primary melanoma; head and neck melanomas had a much greater risk of local recurrence. The Swedish Melanoma Trial and the French Melanoma Trial compared 2-cm versus 5-cm WLE in patients with melanomas less than 2-mm Breslow thickness. Neither trial showed an advantage for a 5-cm margin excision in terms of local recurrence, disease-free survival, or overall survival. The British Collaborative Trial randomized 900 patients with melanomas 2 mm thick or larger to a 1-cm versus 3-cm margin excision; elective lymph node dissection and sentinel node biopsy were not permitted. There were no significant differences in local and in-transit recurrence, disease-free survival, or overall survival. However, there was a marginally significant increase in locoregional recurrence in the group that underwent a 1-cm WLE when local, in-transit, and regional nodal recurrence were considered together. Therefore, for patients with melanomas 2 mm thick or larger, a 1-cm margin is considered inadequate.
Taken together, these studies suggest that a 1-cm margin is sufficient for melanomas 1 mm thick or smaller. For melanomas more than 1 to 2 mm thick, a 1-cm margin may be acceptable, but a 2-cm margin will likely reduce the small risk of local recurrence and is preferred when feasible. For patients with melanomas more than 2 mm thick, a 2-cm margin is appropriate. There are no data to conclude that a 3-cm margin is better than a 2-cm margin. Appropriate margins of excision for thick melanomas (>4 mm thick) have not been studied in the context of randomized trials; however, retrospective analysis has suggested that there is no advantage for margins more than 2 cm. Nonetheless, it may be appropriate to perform wider margin excision for locally advanced melanomas when the risk of recurrence is high.21,22
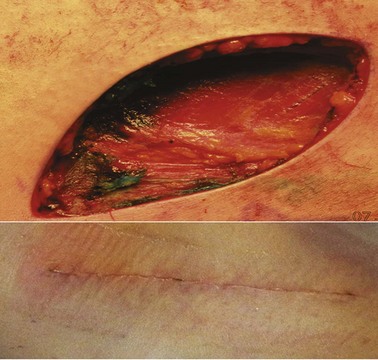
WLE can be performed under local anesthesia in most cases, although general anesthesia is preferred for patients who undergo concomitant sentinel node biopsy or lymphadenectomy. The appropriate margins of excision are measured from the edge of the lesion or previous biopsy scar. Usually, this represents a fusiform incision that encompasses the margins of excision to allow primary closure (Fig. 32-15). WLE is performed to remove the skin and subcutaneous tissue down to the muscular fascia. Excision of the fascia is not necessary in most cases, but may be performed for patients with thick primary tumors. The specimen is submitted for permanent section pathology; frozen section analysis of margins is not performed. In most cases, the incision is closed by mobilizing the skin without the need for complex tissue rearrangement or skin grafting. Complex tissue flaps or skin grafts are rarely necessary, except for melanomas of the head and neck and distal extremities (Fig. 32-16). Tumors arising in proximity to structures such as the nose, eye, and ear may require a compromise of the conventional margins to avoid deformities or disabilities. Subungual melanomas are treated with amputation of the distal digit to provide a 1-cm margin from the tumor. For fingers, ray amputations are unnecessary because the melanoma commonly involves only the distal phalanx; usually, amputation at the distal interphalangeal joint is sufficient. In all cases, resection should achieve histologically negative margins. It should be noted that the recommended margins of excision are the clinically measured margins; it is unnecessary to re-excise the melanoma if the final pathology report indicates that the measured distance from the melanoma to the edge of the excised skin is less than the recommended margin, unless the margin is involved or almost involved by tumor.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree