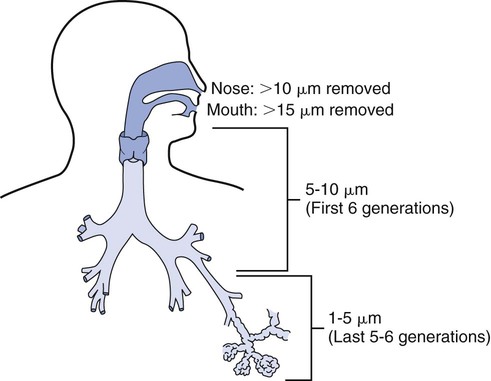
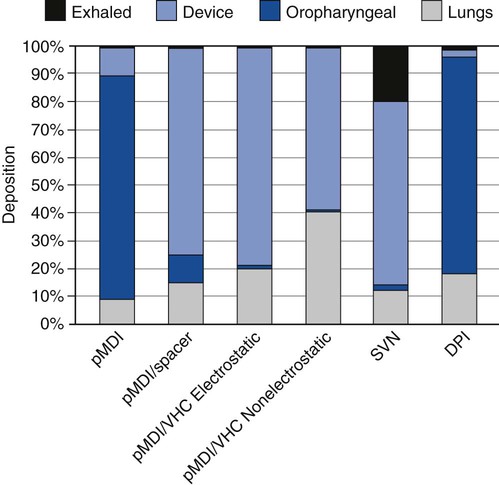
2. Select an appropriate aerosol medication nebulizer on the basis of particle size distributions. 3. Differentiate among the types of aerosol devices. 4. Describe the clinical applications of aerosol devices. 5. Perform therapy using a small-volume nebulizer. 6. Perform therapy using a pressurized metered-dose inhaler. 7. Perform therapy using a dry-powder inhaler. Aerosol generation and delivery to the lungs is a complex topic. Development of both the technology and the scientific basis of inhaled aerosol administration is an ongoing process. Respiratory therapists (RTs) are the primary health care providers responsible for this modality. The term aerosol therapy may be defined as the delivery of aerosol particles to the respiratory tract. At present, the three main uses of aerosol therapy in respiratory care are as follows: 1. Humidification of dry inspired gases, using bland aerosols 2. Improved mobilization and clearance of respiratory secretions, including sputum induction, using bland aerosols of water, and hypertonic or hypotonic saline Lung deposition is dependent on a variety of factors such as the aerosol generator, the patient, the drug, and the disease. Depending on the type of the small-volume nebulizer (SVN) used, most of the drug loss with an SVN occurs in the device, whereas the main drug loss with the pressurized metered dose inhaler (pMDI) and dry-powder inhaler (DPI) occurs in the oropharyngeal airways. Adding a reservoir device to a pMDI or using a nonelectrostatic valved holding chamber shifts the loss from the throat to the reservoir and increases aerosol deposition in the lungs. Figure 19-1 indicates the percentages of drug deposition for different aerosol generators and shows that oropharyngeal loss, device loss, and exhalation or ambient loss differ among aerosol device types, as do lung doses. It should be noted that the overall efficiency in lung deposition of 10% to 15% of the total drug dose is not significantly better than with most pMDI or DPI devices used clinically in the past. Nebulizers, as well as pMDIs and DPIs, are evolving to greater efficiency. One of the major factors influencing aerosol deposition in the lungs is particle size. The effect of particle size on deposition in the respiratory tract is illustrated in Figure 19-2. This chapter will focus on the physical principles of aerosol delivery to the airways and on aerosol-generating devices for the inhalation of drugs. The term nebulizer encompasses a variety of devices that operate on different physical principles to generate an aerosol from a drug solution. The SVN is a type of aerosol generator converting liquid drug solutions or suspensions into aerosol. Because SVNs are often used in infants or in patients in acute respiratory distress, slow breathing and an inspiratory pause may not be feasible or obtainable. One of the main advantages of SVNs is that dose delivery occurs over 60 to 90 breaths rather than in one or two inhalations, as with pMDIs. Thus, a single ineffective breath will not undermine the efficacy of the treatment. Box 19-1 summarizes the advantages and disadvantages of SVNs. SVNs may be classified into three categories: (1) jet (pneumatic) nebulizers, (2) mesh nebulizers, and (3) ultrasonic nebulizers. Box 19-2 lists the nebulizers that have specific applications. The following is the step-by-step process for the use of an SVN. 1. Review the patient’s chart. 2. Verify the physician’s order or the facility’s protocol for standard of care. 3. Obtain, clean, and inspect the appropriate equipment prior to entering the patient’s room. 4. Follow personal protective equipment (PPE) requirements, and observe standard precautions for any transmission-based isolation procedure. 5. Identify the patient using two patient identifiers. 6. Introduce yourself to the patient and to the family. 7. Explain the procedure to the patient and to the family, and acknowledge the patient’s understanding. 8. Perform proper hand hygiene, and put on gloves, mask, and protective eyewear, as appropriate for the procedure. 1. Place the patient in the upright position. 3. Select mask or mouthpiece delivery, 4. Place the medication in the nebulizer. 5. Attach the equipment to the appropriate gas for pneumatic power and proper liter flow (6–10 L/min, depending on the device). 7. Coach the patient to breath slowly through their mouth. 8. Reassess pulse rate, respiratory rate, and lung sounds half way through the treatment. 9. Continue the treatment until the nebulizer sputters. 10. Disconnect the nebulizer from the gas source. 11. Rinse the nebulizer with sterile water and leave it to air-dry or discard it, between treatments. 12. Instruct the patient to rinse their mouth following the treatment. 13. Reassess pulse rate, respiratory rate, and lung sounds after the treatment. 14. Remove the supplies from the patient’s room, and clean the area, as needed. 15. Remove PPE, and perform proper hand hygiene prior to leaving the patient’s room. pMDIs, or MDIs are the most common aerosol generators prescribed for patients with asthma or chronic obstructive pulmonary disease (COPD). These devices are small, pressurized canisters for oral inhalation of aerosol drugs and contain multiple doses of accurately metered medication. The drug in a pMDI is either a suspension of micronized powder in a liquefied propellant or a solution of the active ingredient in a co-solvent (usually ethanol) mixed with the propellant hydrofluoroalkanes (HFAs). Advantages and disadvantages of drug delivery by pMDI are listed in Box 19-3. The conventional pMDI has a press-and-breathe design. When the canister is depressed into the actuator, the drug–propellant mixture in the metering valve is released under pressure. The liquid propellant rapidly expands and vaporizes, or “flashes,” as it ejects from the pressurized valve into ambient pressure. This expansion and vaporization shatters the liquid stream into an aerosol. The initial vaporization of propellant causes cooling of the liquid–gas aerosol suspension, which can be felt if discharged onto skin (with the older versions of pMDIs, chlorofluorocarbons [CFCs] were used); however, HFA versions have a much “warmer” spray temperature. On release, the metering valve refills with the mixture of drug and propellant from the bulk of the canister and is ready for the next discharge. The accuracy and consistency of dose from pMDIs may be more sensitive to handling practices than previously thought. Research on the drug content of sprays of albuterol via pMDIs has shown that various factors affect dose consistency. These factors are listed in Box 19-4. Extension, or reservoir, devices were introduced primarily to simplify the complex coordination of aiming, actuation, and breathing with a pMDI. Figure 19-3 is an illustration of a generic reservoir device. Using accessory devices with pMDIs improves the effectiveness of aerosol drug administration because pMDI accessory devices can modify the aerosol discharged from a pMDI in the following three ways:
Medication Delivery Devices
Equipment
» Skill Check Lists
19-1 Using a Small-Volume Nebulizer Therapy
Procedural Preparation
Implementation
19-2 Using a Pressurized Metered-Dose Inhaler
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Medication Delivery Devices