Chapter 19 Medical Treatment of Peripheral Artery Disease
Treatment of patients with peripheral artery disease (PAD) must take into consideration the risk of adverse cardiovascular events related to systemic atherosclerosis (myocardial infarction [MI], stroke, death) and limb-related symptoms and prognosis (functional capacity, quality of life, limb viability). The risk of MI, stroke, or death related to cardiovascular disease is increased three- to sixfold in patients with PAD (see Chapter 16). Functional limitations imposed by PAD, including symptoms of limb claudication, impaired walking ability, and critical limb ischemia (CLI), adversely affect quality of life and restrict patients’ abilities to participate in many basic vocational and recreational activities. In addition, patients with PAD are at increased risk of lower-extremity ulceration and amputation, and thus foot care represents an important component of management of these patients. Medical management of the patient with PAD has three central goals: (1) prevention of cardiovascular events, (2) improvement of quality of life and functional capacity, and (3) protection and care of the limb (Fig. 19-1). In this chapter, we review the evidence to support aggressive risk factor modification and antiplatelet therapy for patients with PAD to reduce adverse cardiovascular events. We also review the physical and medical therapies used to treat patients with intermittent claudication and CLI to improve lower-extremity function and ameliorate symptoms. Foot care for PAD is briefly discussed. Catheter-based revascularization for PAD is reviewed in Chapter 20, and surgical revascularization for PAD is reviewed in Chapter 21. Multisocietal consensus guidelines for management of the patient with PAD are available and may be helpful in clinical practice.1,2
Risk Factor Modification and Antiplatelet Therapy for Prevention of Cardiovascular Events
Smoking Cessation
Tobacco smoking is strongly associated with development and progression of PAD, with the risk of PAD among smokers as high as threefold that of nonsmokers (see Chapter 16). Smoking cessation is a critical component of risk factor modification for patients with PAD. Epidemiological studies have established that smoking cessation improves both cardiovascular and limb-related outcomes among patients with PAD. Given the established hazards of cigarette smoking, it would be unethical to conduct a randomized clinical trial of smoking cessation.
Smoking cessation has salutary effects on claudication symptoms, exercise physiology, and limb-related outcomes in patients with symptomatic PAD. Patients with intermittent claudication who quit smoking have longer pain-free walking times and maximal walking times compared with patients who continue to smoke.3 In a prospective study of patients with intermittent claudication followed with serial noninvasive vascular testing over a period of 10 months, patients who quit smoking had significant improvements in maximal treadmill walking distance and postexercise ankle pressure, whereas ongoing smokers had no changes in these parameters.4 Smoking cessation is also associated with improved clinical outcomes in patients with PAD. In a Swedish study, patients with intermittent claudication who were active smokers or who had quit within 6 months were followed prospectively for development of limb-related and cardiovascular outcomes.5 At 7 years of follow-up, ongoing tobacco smoking was associated with development of CLI and was also an independent predictor of the need for surgical revascularization. Indeed, only patients who continued to smoke developed rest pain during the follow-up period. Ongoing smoking was also associated with development of MI and a trend toward decreased overall survival at 10 years of follow-up.
Continued cigarette smoking is associated with adverse outcome among patients with PAD referred for vascular surgery. In a prospective study of patients referred for femoropopliteal arterial bypass grafting, ongoing tobacco use was associated with a significant reduction in the 1-year cumulative patency rate of both venous and prosthetic lower-extremity bypass grafts.6 In an Australian study of patients who underwent lumbar sympathectomy or lower-extremity bypass grafting for symptomatic PAD, patients who quit smoking following surgery had dramatically improved 5-year survival rates compared to patients who continued to smoke.7 The majority of deaths that occurred in the postoperative patients were due to a major vascular event, whereas the remaining deaths were due to other smoking-related illnesses, principally chronic obstructive pulmonary disease (COPD) and lung cancer.
Degree of ongoing tobacco use following revascularization may also be predictive of adverse events. In a registry study of patients who underwent their first arterial revascularization procedure, patients categorized as heavy smokers (>15 cigarettes/day) had significantly reduced overall survival compared to moderate smokers (<15 cigarettes/day).8 In addition, there was a 10-fold higher amputation rate among heavy smokers compared with moderate smokers at 3 years’ follow-up.
Despite the multiple benefits of smoking cessation in patients with PAD, it is an extremely difficult goal to accomplish, and initial success rates are low. The efficacy of physician advice in achieving smoking cessation is less than 5%.9 The success rate is at least 10-fold higher when smoking cessation advice and encouragement are given to patients at risk for MI, or patients who have survived an MI. Intensive counseling customized to PAD patients who continue to smoke is associated with a significant improvement in confirmed tobacco abstinence at 6 months of follow-up, compared to standard clinical smoking cessation advice.10 Smoking cessation programs may be more successful when coupled with pharmacological therapy, including both nicotine and non-nicotine agents. The antidepressant bupropion has been demonstrated to improve tobacco abstinence rates at 12 months relative to placebo when used alone or in combination with the nicotine patch.11 Recently, varenicline, a novel partial agonist of the nicotinic acetylcholine receptor (nAchR) α4β, has been shown to improve tobacco abstinence rates among subjects both with and without cardiovascular disease, including patients with PAD.12,13 Among those with cardiovascular disease, varenicline was associated with a threefold likelihood of abstinence at 1-year follow-up compared with placebo, although the absolute abstinence rate was only 19.2%.13 Side effects of varenicline include sleep abnormalities, nausea, and flatulence.13,14 Both varenicline and bupropion are associated with an increased risk of neuropsychiatric side effects. Package labeling for both agents includes a black box warning recommending observation for changes in behavior or mood or development of suicidal ideation while receiving these agents for smoking cessation treatment.14,15
Lipid-Lowering Therapy
Dyslipidemia is a well-established risk factor for development of atherosclerotic vascular disease, including coronary artery disease (CAD) and cerebrovascular disease (CVD). In addition, epidemiological studies have established dyslipidemia—specifically high total and low-density lipoprotein (LDL) cholesterol—as a risk factor for development of atherosclerosis in the peripheral circulation16–18 (see Chapter 16). There is a less established association between low levels of high density lipoprotein (HDL) cholesterol and development of claudication.16,19,20 The association, if any, between elevated triglycerides and PAD is controversial.19,20 The association of dyslipidemia with vascular disease has led to extensive investigation of lipid-lowering therapy as a clinical strategy to prevent myocardial ischemia, stroke, and death in patients with systemic atherosclerosis. Some of these studies specifically addressed the use of lipid-lowering therapy in patients with PAD.
Given convincing epidemiological evidence that dyslipidemia is a risk factor for development of atherosclerosis and subsequent cardiovascular events, multiple randomized clinical trials investigated the use of lipid-lowering agents for prevention of death and other major cardiovascular events in high-risk patients. Development of highly effective and safe agents, particularly HMG-CoA reductase inhibitors (“statins”), has led to widespread application of lipid-lowering therapy for patients with hyperlipidemia and atherosclerotic vascular disease. In recent decades, multiple large randomized controlled trials established the role of lipid-lowering pharmacotherapy, primarily with statins, in secondary prevention of cardiovascular events among patients with CAD.21–24 Although these studies were not designed to specifically investigate the long-term benefit of lipid-lowering therapy in patients with PAD, the findings are of relevance in these patients because most patients with PAD have either symptomatic or asymptomatic coronary atherosclerosis.25–27
The Scandinavian Simvastatin Survival Study (4 S) was the first major clinical trial to demonstrate the survival benefit of aggressive lipid-lowering therapy with statins in hypercholesterolemic patients with CAD.22 In secondary analyses, the 4 S investigators examined the effect of simvastatin on development of symptoms and signs of atherosclerotic vascular disease, including claudication and the appearance of a vascular bruit, during semiannual physical examinations of the study participants.28 There was no significant difference in detection of new or worsening femoral bruits, although this endpoint was likely limited by interobserver variability and the limited sensitivity of the physical examination. There was a 38% reduction in the incidence of intermittent claudication and a 30% reduction in cerebrovascular events among patients randomized to simvastatin.22,28 Similar findings have been confirmed in subsequent studies of the use of HMG-CoA reductase inhibitors for treatment of symptomatic claudication, discussed later in this chapter.
The Heart Protection Study extended use of statins to secondary prevention of cardiovascular events in patients with atherosclerosis in any major vascular bed, and to primary prevention of cardiovascular events in high-risk patients, particularly diabetics.27,29 Patients were randomized to simvastatin or placebo and followed for a mean of 5 years. Eligible patients included those with documented CAD, CVD, or PAD, or patients without documented atherosclerotic vascular disease believed to be at high risk of a major vascular event due to diabetes or multiple cardiac risk factors. Patients enrolled in the study on the basis of PAD had intermittent claudication, had undergone lower-extremity revascularization, or had objective evidence of “leg artery stenosis.” The majority of patients randomized in the study had a history of CAD (65%); of these, 30% had PAD. There was a 13% reduction in the relative risk of all-cause mortality among patients randomized to simvastatin, due largely to a 17% reduction in the risk of vascular death. There was a 24% reduction in the first occurrence of any major vascular event among patients randomized to simvastatin. Patients enrolled on the basis of PAD alone, with no documented CAD, had a 19% reduction in incidence of first major vascular event if randomized to simvastatin. In a subsequent subset analysis of the 4588 subjects with PAD, randomization to simvastatin was associated with a 20% reduction in noncoronary revascularization procedures compared to placebo.30 There was no significant benefit of simvastatin on the incidence of lower-extremity amputation.
Non-LDL cholesterol, particularly low HDL cholesterol, has been identified as an independent risk factor for development of CAD.31,32 Pharmacological agents that raise HDL cholesterol (e.g., fibric acid derivatives, niacin) have not been as widely studied as agents that lower total and LDL cholesterol (i.e., statins) for secondary prevention of cardiovascular events in patients with atherosclerotic vascular disease, and specifically for PAD. The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) investigators randomized men with documented CAD and low HDL cholesterol to gemfibrozil or placebo and found a 22% reduction in the primary composite endpoint of death from CAD or nonfatal MI in the gemfibrozil cohort after a median of 5.1 years of follow-up.21 The incidence of peripheral vascular surgery, the only PAD endpoint studied, was not significantly different within the treatment groups. Cardiovascular benefit has not been found in subsequent studies of other fibrates. The Bezafibrate Infarction Prevention (BIP) study was a randomized study of bezafibrate or placebo in patients with a previous MI or stable angina.33 Bezafibrate did not significantly affect the primary endpoint of fatal or nonfatal MI or sudden death. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a randomized study of fenofibrate or placebo in patients with diabetes.34 Fenofibrate did not significantly affect the primary outcome of fatal coronary heart disease (CHD) and nonfatal MI, but did decrease secondary endpoints such as nonfatal MI and coronary and peripheral revascularization. Given these limited data, the role of therapies to target non-LDL cholesterol in the management of patients with PAD is uncertain.
Recommendations
The American College of Cardiology/American Heart Association (ACC/AHA) PAD guidelines recommend treatment of patients with PAD with an HMG-CoA reductase inhibitor to an LDL goal of less than 100 mg/dL (class I). A lower LDL goal of less than 70 mg/dL is recommended as a more aggressive option for those patients at very high risk of an ischemic event (class IIa).1 Statins should be prescribed for all patients with PAD regardless of whether or not they have documented CAD. To date, no clinical trial has investigated the role of combination therapy (i.e., statin plus fibrate, statin plus ezetimibe) specifically among patients with PAD.
Treatment of Hypertension
Control of hypertension is critical for preventing stroke, MI, and congestive heart failure (CHF). Blood pressure control can be achieved with a number of pharmacological agents, either individually or in combination. Large randomized clinical trials have demonstrated the benefits of pharmacotherapy on clinical outcomes, including death.35,36 Although patients with hypertension and PAD are at increased risk of serious vascular events, few studies have specifically addressed the benefit of blood pressure lowering in this population.
Effect of blood pressure lowering on claudication
Conventional medical wisdom and small clinical trials have alleged that intensive blood pressure lowering, particularly with β-adrenergic blockers, may worsen symptoms in patients with claudication and PAD.37–41 The safety of blood pressure–lowering therapy, specifically with β-adrenergic blockers, in patients with PAD and claudication is of great importance. A substantial percentage of patients with PAD have concomitant CAD. In these patients—in particular, those who have had an MI—treatment with a β-adrenergic blocker has been demonstrated to be lifesaving therapy in large multicenter randomized clinical trials.42,43 β-Adrenergic blockers are also crucial for management of symptomatic angina pectoris in patients with CAD and patients with left ventricular (LV) dysfunction. In addition, β-adrenergic blockers are a key component of perioperative management of patients with PAD and claudication undergoing lower-extremity revascularization surgery.44,45
In a meta-analysis of small randomized controlled clinical trials of the use of β-blockers in patients with PAD and intermittent claudication, there was no significant effect on pain-free or maximal walking distance among patients treated with β-blockers.46 Of the 11 studies included in the analysis, only one study of 20 patients demonstrated an adverse effect of β-blockers on leg symptoms.40 Recently there has been interest in the potential for the β-blocker nebivolol to improve claudication symptoms.47,48 In one study of 128 patients with intermittent claudication randomized to nebivolol or metoprolol for a 48-week treatment period, there was no significant difference in pain-free or maximal walking distance between the two β-blocker groups. There were modest improvements in initial (for nebivolol) and absolute (both nebivolol and metoprolol) claudication distance compared to baseline, but in the absence of a placebo-treated control group, interpretation of these findings is limited.47
Demonstration of the safety of angiotensin-converting enzyme (ACE) inhibitors in the majority of patients with claudication is very important, particularly given the findings of recent trials that have established the importance of this class of agents in preventing cardiovascular events.49 The effect of blood pressure–lowering therapy for 6 weeks with the ACE inhibitor perindopril was studied in patients with hypertension and one of many comorbidities, including PAD.50 A subgroup of patients had significant symptomatic PAD, defined as a pain-free walking distance of 80 to 200 meters (Fontaine IIb) and an imaging study that demonstrated evidence of iliac or femoral arterial occlusive disease. Among the patients with PAD, there was no difference in the pain-free or maximal walking distance between the perindopril and placebo groups, although walking distance increased modestly in both groups above baseline. None of the PAD patients reported worsening claudication. More recently, Ahimastos et al. reported a beneficial effect of the ACE inhibitor ramipril.51 In a pilot study of 40 patients with superficial femoral artery (SFA) occlusive disease and intermittent claudication, without diabetes mellitus or hypertension, there was significant improvement in maximal walking time among patients randomized to ramipril compared with placebo.
Clinical outcomes trials
Two major clinical trials have investigated the potential benefits of blood pressure–lowering therapy in preventing cardiovascular events in patients with PAD. The Appropriate Blood Pressure Control in Diabetes (ABCD) trial studied the effect of intensive blood pressure control compared with moderate blood pressure control on the occurrence of cardiovascular events and renal insufficiency in diabetic patients.52,53 Normotensive (diastolic blood pressure 80-90 mm Hg) and hypertensive (diastolic blood pressure > 90 mm Hg) diabetic patients were enrolled. The hypertensive patients were randomized to receive either enalapril or nisoldipine as first-line therapy. The normotensive patients were randomized to either intensive blood pressure treatment (with further randomization to one of the two study drugs) to achieve a reduction in diastolic pressure of 10 mm Hg, or standard therapy, in which case they received a placebo. Patients were followed for a mean of 5 years. In a substudy of the diabetic patients in the normotensive cohort, the effect of intensive blood pressure treatment among patients with PAD was investigated.53 Among the 220 normotensive patients randomized to intensive blood pressure treatment, there was a 65% reduction in the relative risk of MI, stroke, or cardiovascular death. There was a strong inverse relationship between baseline ankle-brachial index (ABI) and risk of a cardiovascular event. Intensive blood pressure control negated this relationship and normalized the odds of a cardiovascular event toward that of patients with a normal ABI (Fig. 19-2). The benefit of intensive blood pressure control was evident even in patients with a severely decreased ABI, a subset of patients that had been excluded from prior studies.50 There were too few patients with PAD to analyze data for enalapril and nisoldipine separately. The findings of the ABCD trial emphasize the importance of intensive blood pressure control in diabetic patients with PAD.
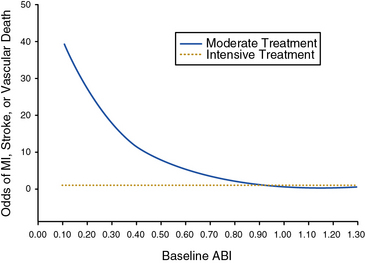
(Reproduced with permission from Mehler PS, Coll JR, Estacio R, et al: Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 107:753–756, 2003.)53
The Heart Outcomes Prevention Evaluation (HOPE) investigators tested the efficacy of the ACE inhibitor ramipril for primary and secondary prevention of cardiovascular events in high-risk patients.49 Patients were enrolled on the basis of established atherosclerotic vascular disease (CAD, prior stroke, or PAD) or diabetes mellitus with additional cardiac risk factors. Some 44% of patients randomized had evidence of PAD, as manifested by a history of claudication with an abnormal ABI of less than 0.8, limb revascularization procedure, amputation, or angiographic evidence of arterial stenosis.54 Patients were randomized to receive ramipril or placebo. At a mean of 5 years of follow-up, there was a 22% reduction in the primary composite endpoint of MI, stroke, or cardiovascular death among patients randomized to ramipril (Fig. 19-3). In subgroup analysis, the benefit of ramipril was present regardless of baseline blood pressure. The benefit of ramipril on the composite endpoint was present among the subgroup of patients with PAD. The benefit of ramipril was likely due to cardiovascular benefits other than its blood pressure–lowering properties because the mean decrease in blood pressure among patients randomized to active therapy was very small. The findings of the HOPE trial indicate that patients with PAD should be treated with an ACE inhibitor, regardless of baseline blood pressure or the presence or absence of diabetes.
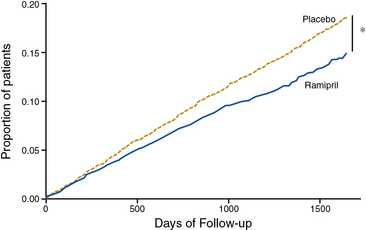
(Reproduced with permission from Yusuf S, Sleight P, Pogue J, et al: Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153, 2000.) 49
For patients with PAD who are intolerant of ACE inhibitors (e.g., development of cough), an angiotensin receptor blocker (ARB) is an acceptable alternative agent for cardiovascular risk reduction. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint (ONTARGET) Trial, 25,620 patients at high risk for cardiovascular events, a population similar to the HOPE trial, were randomized to receive ramipril, the ARB telmisartan, or a combination of the two agents.55 The ONTARGET population included 2468 patients with symptomatic PAD. After a median follow-up of 56 months, the primary cardiovascular event rates in the ramipril, telmisartan, and combination therapy groups were statistically the same (≈︀6.5%). The combination of ramipril and telmisartan, however, was associated with higher rates of renal insufficiency and hypotension than either agent alone.
Recommendations
Blood pressure control is an important component of cardiovascular risk reduction among patients with PAD, and all patients with PAD should routinely undergo blood pressure assessment. Blood pressure should be measured at least once in both upper extremities to exclude the possibility of occult subclavian stenosis leading to inaccurate blood pressure assessment in one of the arms. Any class of antihypertensive agents, including β-blockers, can be safely prescribed for blood pressure lowering, although ACE inhibitors or ARBs should be considered as first-line therapies. Caution should be exercised when managing the patient with CLI because aggressive blood pressure lowering may be detrimental in this small subset of patients. Even normotensive patients with PAD may benefit from ACE inhibitor or ARB therapy. Aggressive blood pressure control is particularly important for diabetic patients with PAD. According to the ACC/AHA PAD guidelines, target blood pressure for patients with PAD is below 140/90 mm Hg for nondiabetic and below 130/80 for diabetic patients (class I recommendation).1
Control of Diabetes Mellitus
Multiple epidemiological studies have established a strong association between diabetes mellitus and PAD16,17,56,57 (see Chapter 16). The relative risk of PAD is two to four times that of nondiabetic patients. Presence of diabetes is associated with adverse limb-related outcomes among patients with documented PAD, including the need for amputation.58,59 In the Strong Heart Study, worsening glycemic control (determined by hemoglobin A1c [HbA1c] level) was associated with increased incidence of lower-extremity amputation among diabetic Native Americans with and without PAD.60 In addition, diabetes is associated with a markedly increased risk of a major cardiovascular event, including myocardial ischemia, stroke, and death. Therapeutic options for achieving glycemic control in diabetic patients include insulin, sulfonylureas, metformin, the thiazolidinediones (“glitazones”), and novel agents that modify carbohydrate absorption and breakdown into glucose (α-glucosidase inhibitors) or increase insulin bioavailability through differing mechanisms (e.g., repaglinide, nateglinide, sitagliptin).61 Whereas clinical trials have established the vital importance of rigorous glycemic control for preventing microvascular complications in diabetic patients, the benefits of glycemic control for prevention of major cardiovascular events has not been definitively established. In addition, recent randomized controlled trials have indicated that certain high-risk patients may be more likely to experience adverse effects with very intensive glycemic control.62
Clinical outcomes trials
The Diabetes Control and Complications Trial (DCCT) Research Group investigated whether intensive glycemic control could improve clinical outcomes in diabetic patients.63 A total of 1441 young patients (aged 13-39 years) with type 1 diabetes mellitus, with and without retinopathy, were randomized to one of two glycemic control strategies: (1) intensive therapy with an insulin pump or frequent injections to maintain blood sugar as close to normal as possible, or (2) conventional therapy with once- or twice-daily insulin injections. Development or progression of retinopathy was the primary endpoint of the study, and the low cardiovascular risk profile of the study population reflected this goal. Peripheral artery disease endpoints included development of claudication, persistent loss of a pedal pulse, and need for a revascularization procedure or limb amputation. Patients were followed for a mean of 6.5 years. Among patients randomized to intensive glycemic control, there were significant reductions in development and progression of retinopathy and proteinuria and development of sensorimotor neuropathy compared to patients treated with conventional glycemic control. Owing to the young average age of the patient population, there were few deaths or major macrovascular events in either treatment group. Nonetheless, randomization to intensive glycemic control was associated with a nonsignificant 42% reduction in peripheral vascular and coronary events.64 Perhaps more importantly, however, randomization to intensive glycemic control was protective against development of elevated total and LDL cholesterol—risk factors for future development of cardiovascular events in this young patient population. Long-term follow-up data from the DCCT study were recently published.65 Additional observational follow-up data were available for 93% of patients enrolled in the original trial in the Epidemiology of Diabetes Interventions and Complications study. At a mean follow-up of 17 years, randomization to intensive glycemic control showed a persistent 42% reduction in risk for a major cardiovascular event.
The United Kingdom Prospective Diabetes Study 33 (UKPDS 33) was designed to complement the findings of the DCCT by investigating the effect of intensive glycemic control (with sulfonylureas or insulin) on the incidence of macrovascular events in type 2 diabetic patients.66 An earlier study of glycemic control strategies in type 2 diabetic persons found no cardiovascular benefit of intensive therapy with insulin and an excess of cardiovascular deaths among patients treated with the sulfonylurea tolbutamide.67 This early finding generated concern regarding the safety and potential cardiovascular toxicity of sulfonylureas with prolonged use in diabetic patients. In the UKPDS 33, patients were randomized to intensive therapy with insulin or one of three sulfonylureas, or to conventional treatment with a prescribed diabetic diet; they were followed for a median of 10 years.66 Although there were no differences in the treatment groups with regard to total mortality, cardiac mortality, or diabetes-related mortality, there was a 16% reduction in the relative risk of MI in the group randomized to intensive therapy. There was also a 39% reduction in relative risk of amputation in the intensive therapy group, which did not achieve statistical significance. There were too few deaths attributable to PAD to allow for comparison. Consistent with the findings of the DCCT, there was a highly significant 25% reduction in relative risk of microvascular complications among patients randomized to intensive glycemic control, including the need for retinal surgery.
A follow-up study evaluated a subset of participants from UKPDS 33 annually, either with clinical office visits or questionnaires, for up to 10 years after completion of the trial.68 During this period of follow-up, patients were managed at the discretion of their physicians rather than per trial protocol, and differences in HbA1c levels between the intensive and conventional treatment groups equalized. Despite this finding, the significant risk reduction in microvascular events among patients initially randomized to intensive therapy persisted (24% reduction). More importantly, there were statistically significant gains in terms of demonstration of delayed cardiovascular benefit of intensive therapy of diabetes, with reductions in all-cause mortality (27% reduction) and risk of MI (33% reduction). There was no significant reduction in risk of stroke or peripheral vascular outcomes.
There has been great interest in use of the insulin-sensitizing agents, metformin and the thiazolidinediones (glitazones), for prevention of cardiovascular events in diabetic patients. In a substudy of the UKPDS (UKPDS 34), newly diagnosed, overweight, type 2 diabetic patients were randomized to intensive therapy with metformin or a prescribed diabetic diet.69 Patients were followed for a median of 10.7 years. In contrast to the sulfonylurea arm of the study, there was a significant 42% reduction in diabetes-related mortality and a 36% reduction in all-cause mortality among patients randomized to metformin therapy compared with patients randomized to diet. There was no difference in the incidence of PAD endpoints between the two groups, although the total number of clinical events related to PAD was small.
Use of thiazolidinediones for glycemic control and prevention of cardiovascular events among diabetic patients has been an area of recent study and significant controversy. In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study, 5238 type 2 diabetic patients with established macrovascular (atherosclerotic) disease, including CAD, prior stroke, or PAD as defined by history of intermittent claudication (with abnormal ABI or toe-brachial index [TBI]) or prior major amputation were randomized to receive pioglitazone or placebo atop their established diabetes medical regimen and followed for incident cardiovascular events.70 At a mean follow-up of nearly 6 years, there was no significant difference between pioglitazone- and placebo-treated patients in the primary composite endpoint of all-cause mortality, nonfatal MI, stroke, acute coronary syndrome (ACS), coronary or leg endovascular or surgical revascularization, and amputation above the ankle. There was a 16% reduction in the secondary composite endpoint of death, nonfatal MI, or stroke in patients randomized to pioglitazone.
Recently, there have been safety concerns related to the safety of rosiglitazone, another thiazolidinedione, including risk of MI and congestive heart failure.71 The RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes) trial randomized type 2 diabetic patients on sulfonylurea or metformin therapy with adequate glycemic control to receive added rosiglitazone therapy or added sulfonylurea and metformin in an open-label design.72 After a mean of follow-up of 5.5 years, there was no significant difference in the composite endpoint of cardiovascular death or hospitalization between the rosiglitazone and sulfonylurea/metformin combination therapy groups. There was, however, a more than twofold increase in risk of significant CHF (requiring hospitalization or leading to death) among rosiglitazone-treated patients. On the basis of previous meta-analyses and the findings of the RECORD trial, there is now a black box warning on the rosiglitazone label regarding adverse cardiovascular effects, and prescriptive access to this medication is restricted by the drug manufacturer.73
Three randomized controlled trials that have investigated the benefit of very intensive glycemic control (target HbA1c 6%-6.5% or normoglycemia) versus modern standard glycemic control (target HbA1c <7%-7.5%) among patients with type 2 diabetes.74–76 In the Action and Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, there was a 14% reduction in microvascular events among patients randomized to highly intensive therapy, but there was no significant reduction in macrovascular events, including risk of death, MI, or stroke.76 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, not only was there no benefit of highly intensive glycemic control on a composite endpoint of cardiovascular death or nonfatal MI or stroke, but there was also an increased risk of death from any cause (22%) or cardiovascular death (35%) among individuals randomized to highly intensive therapy.74 There was a marked increase in hypoglycemic episodes requiring medical assistance (10.5% vs. 3.5%) among patients randomized to highly intensive therapy. The ACCORD trial was terminated early at the recommendation of its data safety and monitoring board based upon these findings. Finally, the Veterans Affairs Diabetes Trial (VADT) randomized type 2 diabetes patients to either standard therapy or highly intensive glycemic control (goal HbA1c <6%) using rosiglitazone plus either glimepiride or metformin, based upon body mass index (BMI; metformin for patients with BMI ≥ 27 kg/m2).75 In this trial, there was no benefit of intensive glycemic control on the risk of macrovascular, or surprisingly microvascular, complications during 5.6 years of follow-up.
Recommendations
The American Diabetes Association has published guidelines for the medical care of diabetic patients with and without PAD.77,78 The ACC/AHA PAD guidelines also address care of the diabetic patient with PAD.1 In response to the findings of ACCORD, ADVANCE, and VADT, updated multisocietal guidelines for glycemic control have been published.79 The standard target HbA1c of less than 7% stands as a recommendation for most patients. However, it is recommended that less stringent glycemic control be considered for patients with prior history of severe hypoglycemic reaction, for those with extensive micro- and macrovascular disease, and for those with limited life expectancy. A more stringent target (e.g., HbA1c <6%-6.5%) is recommended as a target for healthier patients with a long life expectancy and no major cardiovascular disease.
In addition to glycemic control, periodic foot examination by healthcare providers and patient education regarding preventive foot care is critical for diabetic patients with PAD, particularly given the prevalence of peripheral neuropathy. The American Diabetic Association and the ACC/AHA recommend ABI measurement for all diabetic persons older than 50 years of age and for some younger patients with additional risk factors.1,77
Treatment of Hyperhomocysteinemia
Hyperhomocysteinemia is a disorder associated with derangements of the metabolic pathway involved in metabolism of the essential amino acid methionine.80 Hyperhomocysteinemia is due to an inherited defect of one of the enzymes involved in transsulfuration or remethylation of homocysteine, or can also occur as a result of malnutrition and deficiency of key cofactors for these enzymatic reactions (i.e., folic acid, vitamins B6 and B12). Hyperhomocysteinemia is associated with end-stage renal disease, although the mechanism by which this occurs is not well established.80 Inherited homocystinuria, the most striking form of hyperhomocysteinemia, is due to homozygous deficiency of the enzyme cystathionine β-synthase, a critical enzyme of the transsulfuration pathway of homocysteine. Homocystinuria is associated with mental retardation, ectopia lentis, and premature coronary and peripheral atherosclerosis. Among heterozygotes for cystathionine β-synthase deficiency, homocysteine levels are significantly lower (on the order of 20-40 μmol/L vs. up to 400 μmol/L for homozygotes), although there is also a predisposition to premature atherosclerosis.80 Multiple epidemiological studies have established an association between elevated plasma homocysteine levels and development and progression of CAD and CVD. Extensive epidemiological evidence has also established hyperhomocysteinemia as an independent risk factor for asymptomatic PAD and the clinical progression of symptomatic PAD.81–84 In addition to premature atherosclerosis, elevated levels of homocysteine are associated with a hypercoagulable state characterized by a tendency toward venous and arterial thrombosis.80,85
Because elevated plasma homocysteine levels are associated with low levels of the enzymatic cofactors folic acid, vitamin B6, and vitamin B12, vitamin supplementation is an obvious therapeutic consideration for treating hyperhomocysteinemia. One small trial of healthy siblings of patients with hyperhomocysteinemia and premature atherosclerotic vascular disease (PAD, CAD, or CVD before age 56) randomized siblings to combination vitamin therapy with 5 mg folic acid and 250 mg vitamin B6 or placebo.86 Siblings with and without evidence of hyperhomocysteinemia were enrolled. Subjects continued therapy for a period of 2 years and were followed for development of CAD (abnormal stress test), PAD (abnormal ABI or lower-extremity arterial duplex scan), and CVD (abnormal carotid artery duplex scan). Among patients randomized to vitamins, fasting plasma homocysteine levels fell from 14.7 to 7.4 μmol/L (a nearly 50% decline); there was an 18% decline in the placebo group. There was no significant difference in the development of PAD or asymptomatic CVD among the two groups. Among the patients randomized to aggressive vitamin therapy, there was a decrease in the incidence of an abnormal stress test.
Although the effectiveness of supplementation with folic acid and vitamin B12 for lowering plasma homocysteine levels has been established, few data show a benefit of vitamin supplementation to prevent vascular events in patients with hyperhomocysteinemia or established vascular disease. In the second Heart Outcomes and Prevention Evaluation (HOPE-2) trial, patients with atherosclerotic vascular disease were randomized to receive folic acid, vitamin B6 and B12, or placebo and followed for incident cardiovascular events.87,88 Less than 10% of patients in the study population of 5522 had known symptomatic PAD, defined as intermittent claudication or a history of bypass surgery or lower-extremity angioplasty.88 After 5 years of follow-up and despite a mean reduction in plasma homocysteine of 2.4 μmol/L in the vitamin-treated group (compared to an increase of 0.8 μmol/L in the placebo group), there was no benefit of vitamin therapy on the primary composite outcome of fatal or nonfatal MI or stroke.
In the Norwegian Vitamin Trial (NORVIT), patients with acute MI within the preceding 7 days were randomized to one of four arms in a factorial design (placebo, vitamin B6, folic acid + vitamin B12, or combination-therapy folic acid and vitamins B6 and B12).89 There was a 27% reduction in plasma homocysteine levels among individuals who received folic acid and vitamin B12. However, with a median follow-up of 40 months, there was no benefit of folic acid plus vitamin B12 therapy, with or without vitamin B6, on the composite cardiovascular endpoint. Indeed, there was a trend toward increased risk of a fatal or nonfatal cardiovascular event among individuals randomized to triple-vitamin therapy compared to placebo. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial assessed the effect of folic acid and vitamin B12 on vascular outcomes in 12,064 survivors of MI.90 Treatment with these vitamins reduced homocysteine by 3.8 μmol/L (28%) but did not affect the primary outcome of first major vascular event, defined as a major coronary event, fatal or nonfatal stroke, or noncoronary revascularization.
Antiplatelet Therapy
The pivotal role of antiplatelet agents, particularly aspirin, in the secondary prevention of death and MI in patients with CAD has been established by large randomized clinical trials.91,92 The role of antiplatelet therapy in the management of patients with PAD continues to evolve, with important new data gleaned from recent large clinical trials directed primarily at the prevention of cardiovascular events in patients with and without CAD.
A recent meta-analysis of six primary prevention trials of 95,000 individuals at low to average cardiovascular risk (representing 660,000 person-years) found that aspirin reduced the risk of any vascular event by 12%.93 This was due primarily to a decrease in the risk of nonfatal MI, but not stroke or vascular mortality. This meta-analysis also included 16 secondary prevention trials of 17,000 individuals at high-average risk, representing 43,000 person-years. In the secondary prevention trials, aspirin reduced serious vascular events by 29%, including total stroke and coronary events, and was associated with a borderline nonsignificant 9% reduction in vascular mortality.
In 2002, the Antithrombotic Trialists’ Collaboration updated the 1994 meta-analysis of the evidence supporting the use of antiplatelet agents in the management of high-risk patients with atherosclerotic vascular disease.91 Studies with different antiplatelet agents (e.g., aspirin, dipyridamole, picotamide, ticlopidine) were included. The initial meta-analysis determined that antiplatelet therapy significantly reduced the odds of a major vascular event among high-risk patients with atherosclerosis by 27%.92 Also, antiplatelet therapy was effective for preventing both coronary and peripheral artery bypass graft occlusion, with a reduction in relative risk of graft occlusion of approximately one third.94 In the updated analysis, a total of 287 randomized trials with more than 200,000 patients were included.91 Confirming the original findings, there was a 22% reduction in the odds of a serious vascular event (vascular death, nonfatal MI, or nonfatal stroke) among high-risk patients treated with antiplatelet agents. The benefit appeared to be greatest among studies that enrolled patients on the basis of high-risk criteria such as prior or acute MI, PAD, or atrial fibrillation. Among the 42 studies that enrolled 9214 high-risk patients on the basis of PAD, there was a 23% reduction in the odds of a major vascular event among patients randomized to antiplatelet therapy. The benefit of antiplatelet therapy was consistent across all PAD enrollment criteria, including intermittent claudication and surgical lower-extremity revascularization. Of note, 2304 patients in this category were enrolled in a study that randomized PAD patients to treatment with a nonaspirin antiplatelet agent, picotamide, or placebo.95 Picotamide is an antiplatelet agent that both inhibits thromboxane A2 (TxA2) synthase and antagonizes the TxA2 receptor of platelets. It is also noteworthy that none of the trials in this meta-analysis investigated the benefit of aspirin alone (i.e., not in combination with dipyridamole) in standard clinical dosage (81-325 mg daily ) versus placebo, a fact that was highlighted by an updated meta-analysis of aspirin therapy for PAD by Berger et al. in 2009 (discussed later).96
The Critical Limb Ischaemia Prevention Study (CLIPS) randomized patients with ABI less than 0.85 or TBI less than 0.6 who were asymptomatic or had stable leg claudication (Fontaine stage I/II) to aspirin 100 mg daily or placebo.97 Although an initial sample size of 2000 patients was planned, the trial was terminated early because of poor enrollment, and only 366 patients were randomized, with a minority followed for more than 2 years. Aspirin was associated with a 64% reduction in the relative risk of fatal and nonfatal vascular events and a 58% reduction in fatal and nonfatal vascular events or critical limb ischemia.
In contrast to the CLIPS trial, which included patients with clinically significant PAD (claudication or asymptomatic PAD with low ABI), there have been two large randomized clinical trials that investigated the efficacy of aspirin in low-risk asymptomatic PAD patients. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial randomized 1276 patients older than 40 years of age who had type 1 or 2 diabetes mellitus and an “abnormal” ABI of less than 1.0, but who had no known cardiovascular disease and no symptoms of PAD.98 Subjects were randomized to aspirin 100 mg daily or placebo, as well as antioxidant vitamins, in a 2 × 2 factorial design. It is important to note that this was a population that was not only asymptomatic in terms of PAD but also had relatively mild disease, with a median ABI in the study population of 0.9, barely at the lower limit of normal. After a median follow-up of 6.7 years, there was no difference between the aspirin and placebo groups in the rate of fatal or nonfatal vascular events or amputation. In subset analysis, there was a nonsignificant trend toward benefit of aspirin among patients with ABI less than 0.9.
The largest trial of aspirin therapy for prevention of cardiovascular events among patients with PAD to date was the Aspirin for Asymptomatic Atherosclerosis (AAA) Trial.99 In this trial, nearly 29,000 Scottish patients between the ages of 50 and 75 years who had no cardiovascular disease were offered a screening ABI examination. A total of 3350 individuals with ABI below 0.95 were randomized to receive 100 mg aspirin daily or placebo and followed for an average of 8.2 years. In this study, the lower of two ankle pressures was used for calculating the ABI. There was no statistically significant difference in occurrence of the primary composite endpoint of fatal and nonfatal coronary events, stroke, or revascularization between aspirin- and placebo-treated patients (Fig. 19-4).
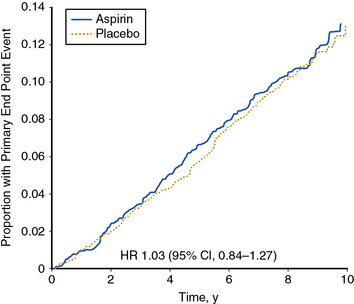
(Reproduced with permission from Fowkes, FG, Price JF, Stewart, MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle-brachial index: a randomized controlled trial. JAMA 303:841–848, 2010.)99
Berger et al. performed a meta-analysis of 18 trials comprising 5269 patients with PAD that evaluated the efficacy of aspirin (± dipyridamole) for prevention of cardiovascular events including nonfatal MI, nonfatal stroke, and cardiovascular death.96 This meta-analysis included the trials incorporated into the Antithrombotic Trialists’ Collaboration 2002 meta-analysis, as well as new data from the POPADAD trial and CLIPS; the AAA study was not included. Aspirin therapy, with or without dipyridamole, did not significantly reduce the rate of cardiovascular events. In subset analyses, aspirin was associated with a significant reduction in the incidence of nonfatal stroke, but not cardiovascular mortality, MI, or major bleeding.
Large multicenter randomized clinical trials have investigated the use of the antiplatelet agent clopidogrel for secondary prevention of cardiovascular events. Clopidogrel is a thienopyridine derivative that inhibits platelet aggregation by antagonism of the adenosine diphosphate receptor.100 Clopidogrel is less likely to cause serious adverse hematological side effects, particularly neutropenia and thrombotic thrombocytopenic purpura, than its analog ticlopidine. Pooled analysis of early clinical trials suggested a trend toward a marginal benefit of ticlopidine over aspirin in the prevention of MI, stroke, or vascular death.92
The Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) study built on this finding and investigated the benefit of clopidogrel versus aspirin in the secondary prevention of cardiovascular events.101 Patients with recent MI (within 35 days), ischemic stroke (within 6 months), or symptomatic PAD were randomized to clopidogrel (75 mg daily) or aspirin (325 mg daily). Patients enrolled on the basis of PAD had intermittent claudication and an abnormal ABI (ABI ≤0.85) or had undergone leg amputation or revascularization. A total of 19,185 randomized patients were followed for development of the primary composite endpoint of first occurrence of ischemic stroke, MI, or vascular death. After a mean 1.9 years of follow-up, there was an 8.7% relative risk reduction in the annual event rate of the primary endpoint among patients randomized to clopidogrel. The benefit of clopidogrel over aspirin was greatest among the subgroup of patients enrolled on the basis of symptomatic PAD, with a relative risk reduction of the composite endpoint of 23.8% (Fig. 19-5). There was no increase in minor or major bleeding episodes associated with clopidogrel, although there was an increased incidence of gastrointestinal hemorrhage among patients randomized to aspirin.
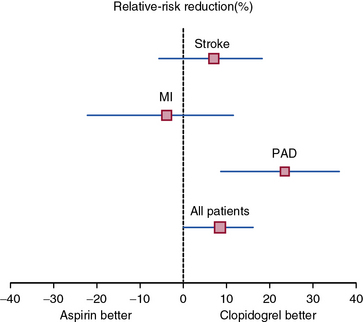
(Adapted and reproduced with permission from the CAPRIE Steering Committee: A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events [CAPRIE]. Lancet 348:1329–1339, 1996.)101
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree