INDICATIONS
Standard Indications
Cervical mediastinoscopy has been originally thought up by an otorhinolaryngologist as a means of excluding patients with unfavorable prognosis from further resectional treatment of lung cancer in times when no computerized tomography existed. Nonselective application created vast numbers of patients undergoing mediastinoscopy and established the procedure as very safe from the statistical point of view, albeit with rarely occurring and notoriously discussed fatal complications.
This classical indication for nodal staging of lung cancer remains to be the most frequent reason for performing mediastinoscopy. Although limited to nodal stations adjacent to trachea and main bronchi in its standard form, diagnostic accuracy superior to imaging studies has long been keeping mediastinoscopy on the throne of gold standard. Hundred percent reliability of staging by mediastinoscopy cannot, by definition, be reached for two obvious reasons: Not all N2 mediastinal nodal stations are accessible by the technique; and tumor-related changes may be located in the nonbiopsied part of the node or in another node of the same station. The first limitation can be overcome by utilizing extended mediastinoscopy (stations 5 and 6), a combination of EUS/EBUS (stations 3p, 8, 9), or VATS (stations 3a, 5, 6, 8, 9). Avoiding the second limitation constitutes the core idea of supermediastinoscopies (TEMLA and VAMLA)—only removing entire nodes and removing all of them can provide complete mediastinal nodal staging.
Endoluminal needle staging techniques had to prove and have proven their usefulness by comparison to mediastinoscopy. High specificity and low negative predictive value make these techniques ideal for detection but insufficient for exclusion of mediastinal disease. Staging algorithms have evolved that utilize the initially concurring endoscopic and surgical techniques as complementary—based on initial imaging studies the invasive staging methods are applied selectively to reach the best possible sensitivity and predictive values (Fig. 4.1).

Figure 4.1 Staging algorithm with selective utility of mediastinoscopy. *Not clearly stated in the guidelines: If suspicion persists after negative EBUS/EUS, mediastinoscopy may be performed; otherwise proceed to surgery. Performing mediastinoscopy in EBUS/EUS negative patients with normal N2 nodes on PET/CT little or no value to staging accuracy. Based on ACCP Guidelines 2013.
Although not clearly supported by prospective trials, surgery after induction treatment is generally accepted as a valid therapeutic option for stage IIIA NSCLC if nodal downstaging can be proven by tissue analysis. Remediastinoscopy, although not widely performed, remains a reliable restaging tool in these patients.
Mediastinal lymphadenopathy unrelated to lung cancer is mostly successfully evaluated by endoluminal needle techniques but occasionally may require mediastinoscopy, which offers highly reliable results for tissue diagnosis of granulomatous diseases, lymphomas, or metastatic spread of extrathoracic malignancies. Tumors accessible to mediastinoscopy may require analysis of larger tissue samples not obtainable by core-needle biopsy.
Contraindications
All contraindications of mediastinoscopy are relative.
The inability to extend the neck for whatever reason may be problematic not only for insertion of the scope but also for manipulation via its channel due to patient’s chin obstructing the route. This can mostly be overcome by rotation of the head to one side.
Previous sternotomy is not a contraindication per se but may become a problem in case of hemorrhagic complications, as the left innominate vein, superior vena cava (SVC), the ascending aorta or pericardium may adhere to the posterior aspect of sternum and suffer damage during emergency resternotomy. Previous aortic arch surgery or aneurysm might represent a relative contraindication as well.
Previous tracheostomy, definitive, persisting, or healed, while isolated from the neck incision, does not represent an absolute contraindication.
Large retrosternal goiter may be removed via transcervical access and mediastinoscopy for exploration of peritracheal pathology may be done in the same session.
All previous interventions in mediastinum, but especially in peritracheal regions, can lead to scarring and difficult dissection (previous mediastinoscopy, tracheal resection, drainage or vacuum-assisted closure for anterior mediastinitis, previous lung resection with en bloc mediastinal lymphadenectomy especially on the right) with the occasional need to abandon mediastinoscopy. Previous esophagectomy with retrosternally placed conduit can hinder the access to anterior tracheal wall.
SVC syndrome is not a contraindication for mediastinoscopy if appropriate precautions are taken.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Clinical examination relevant to mediastinoscopy consists of verification of sufficient neck extension, neck palpation to exclude peripheral lymphadenopathy, presence of goiter, and high-riding innominate artery pulsating in the jugular notch. Tracheal deviation may also be detected by palpation. Subclinical SVC syndrome can yield a positive Pemberton test.
The surgeon should be acquainted in detail with the mediastinal anatomy of the patient and eventual variations and anomalies. Contrast-enhanced CT provides not only data on size and location of lymph nodes or tumors, but also sufficient information on great vessels, that is, high-riding innominate artery, Bovine arch anatomy (left common carotid coming off innominate artery), lobus venae azygos and varicose dilation of the azygos vein in patients with portal hypertension, aneurysm or atheromatous changes of aortic arch and its arteries, and presence of SVC compression or thrombosis. Three-dimensional reconstruction may even identify aberrant bronchial arteries. CT enables better planning of the procedure in the rare case of situs inversus viscerum. Tracheal deviation and compression and esophageal dilation are also relevant for the mediastinoscopist. Presence and extent of retrosternal goiter and its potential conflict with access to peritracheal areas can be evaluated.
Cross-matching blood units preoperatively can save time in case of catastrophic bleeding. Major vascular access and arterial blood pressure monitoring are recommended for patients evaluated as ASA3 or worse and those who could undergo lung resection during the same session if frozen sections are reliably negative. Sternotomy and thoracotomy set must be ready in the room or on the side table. An assistant should be present in the theater or rapidly available for eventual emergencies.
 SURGERY
SURGERY
Positioning
One arm is abducted and provided with intravenous access, preferably on the side contralateral to tumor. Upon intubation the patient is left in supine position with a gel cushion or a soft roll placed directly under the shoulder blades (i.e., level Th5) to extend the neck and a stabilizing cushion supporting the occipital region to prevent spontaneous rotation. The patient is positioned with the head as near the “cranial” edge of the table to keep the working conditions ergonomic. Orotracheal tube is kept in the corner of the mouth on the side of the abducted arm.
The table is moved into slight anti-Trendelenburg position until proximal part of sternum lies horizontally to improve the working angle of the mediastinoscope and instruments inserted.
The operating field is prepared and draped at the level of cricoid cartilage proximally, xiphoid process distally, and midclavicular lines laterally. This allows for a rapid midline sternotomy when bleeding from large systemic arteries occurs. Other vascular injuries requiring additional or stand-alone thoracotomy can be temporarily controlled by gauze packing until the patient is repositioned.
Figure 4.2 depicts one of the possible arrangements of the patient and the operating team.
Rigidity of cervical spine causing the chin get in the way of mediastinoscope can be overcome by gentle rotation or reclination. Some surgeons prefer to sit mainly to get a comfortable view directly through the scope. Video-mediastinoscopy can be done completely without looking through the instrument and therefore the ergonomics are better while standing.
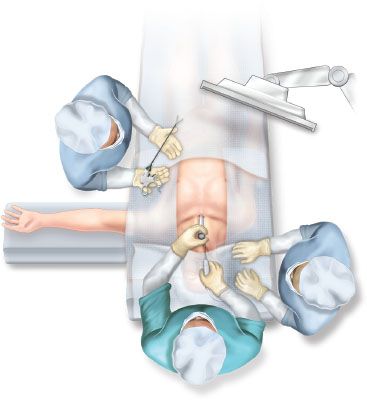
Figure 4.2 Operating room setup for videomediastinoscopy.
Right-handed surgeons hold and move the scope around with their left hand while dissecting in mediastinum. A plastic bag for the suction devices (suctioning electrode, large-bore suction tube) is placed on the side of the dominant hand. Mediastinoscope positioning arm is attached to the table on the nondominant side of the surgeon.
Instruments
The classical Carlens mediastinoscope is 6 cm shorter than modern mediastinoscopes and slightly (1.2 mm) narrower in diameter. Some surgeons prefer this scope for better maneuverability especially in fibrotic mediastinum and in patients with a short or rigid neck.
Modern videomediastinoscopes (Dahan–Linder by Wolf, Huertgen by Storz) have variably long blades (16 cm, 19 cm) that can be distracted parallelly or divergently to create space for bimanual manipulation with increased mobility of instruments.
Dahan–Linder mediastinoscope by Wolf (Richard Wolf GmbH, Knittlingen, Germany) can be fully dismantled, and its dorsal blade has an atraumatic lip protruding dorsally and beyond the distal end. Light cable is attached separately on the right side of the scope, and camera head to the universal camera connector at the top of the handle.
Linder–Huertgen videomediastinoscope by Storz (KARL STORZ GmbH & Co.KG, Tuttlingen, Germany) has both the camera and the light connector integrated in the compact handle and its dorsal blade is straight at the distal end with no protrusions.
Choosing the make and manufacturer of the scope is a matter of personal preference.
Video- Versus Conventional Mediastinoscopy
Videomediastinoscopy has undoubtedly many advantages over conventional mediastinoscopy. The field of vision is extended and magnified 17 times, which combined with high-definition resolution brings far more detail to the eye of the surgeon. Thus, the dissection, hemostasis, and lymph node biopsy can be more detailed, accurate, and extensive.
Ergonomics are improved when sitting to look directly through the scope, however the surgeons arms have to be elevated and his neck stretched which may lead to overstraining and discomfort during longer procedures. Standing during video-mediastinoscopy keeps the susceptible axial skeleton in a more physiologic position even during bimanual operation.
Videomediastinoscopy provides a better access to and better biopsies of the posterior segment of subcarinal nodes. Also LNR paralysis is less frequent with videomediastinoscopy.
Conventional mediastinoscopy might be beneficial in tight mediastinum as the scope is slightly narrower and shorter, which allows for better mobility. For a surgeon uncomfortable with mediastinal anatomy the 3D view through the scope may improve performance.
Best evidence literature search found higher lymph node yield, better sensitivity, and better negative predictive value with videomediastinoscopy.
Technique
Mediastinoscopy starts with a 3- to 4-cm transverse incision 1 fingerbreadth cranial to jugular notch. Placing the incision too low may cause a spatial conflict of the scope with the manubrium and its limited mobility.
As the circumference of the scope averages centimeter at its widest part, it is advisable to make the incision somewhat longer so as to avoid pressure necrosis or burning of the skin. Covering the operation field with adhesive foil will also prevent inadvertent electric burns and allow for safe digital exploration and dissection in the mediastinum without increasing the risk of contamination.
After dividing the platysma muscle (rarely defined near midline) the deep cervical fascia investing the strap muscles is divided in midline and the strap muscles are retracted laterally. Anterior jugular veins and lower thyroid vessels may be retracted laterally as well or transected. A high-riding innominate artery or the left innominate vein may be encountered and their injury should be avoided. Anterior surface of the trachea covered with pretracheal fascia becomes visible caudal to the thyroid isthmus. Here the highest mediastinal nodes are occasionally encountered and may be easily removed along with fatty tissue.
Upon lifting it gently with forceps the pretracheal fascia is incised with scissors and bluntly separated from the trachea so that the index or middle finger can be inserted into this virtual space. Blunt digital dissection along the anterior surface of the trachea follows. The cartilaginous tracheal rings are felt on the dorsal aspect of the finger while the pulsatile innominate artery crossing ventrally, and the medial aspect of the aortic arch are palpated with the palmar aspect.
The pretracheal (aka perivisceral) fascia in mediastinum encompasses the trachea and esophagus all the way to tracheal bifurcation where it becomes continuous with the fibrous pericardium anteriorly but also extends along the bronchi and surrounds bronchial arteries, lymphatic vessels, and subcarinal lymph nodes. It continues below the bifurcation as the investing fascia of the esophagus. The peritracheal nodes lie outside this fascia, therefore, upon safely entering the mediastinum by keeping the innominate artery outside the finger dissection field, the perivisceral fascia has to be bluntly or sharply penetrated and widely open caudal to innominate artery all the way to bifurcation (Fig. 4.3). To reach the subcarinal nodes, the perivisceral fascia has to be penetrated again under vision at the level of bifurcation, as these nodes lie within the space defined by the fascia.
Digitally opening the perivisceral fascia enables the surgeon to assess the location, size, and consistence of nodes present. Slight lateral mobilization at the level of innominate artery improves the mobility of the scope and decreases the risk of severe compression or laceration of the vessel. Any resistance encountered might be caused by entering the wrong plane or by pressing against great vessels or lymph nodes.
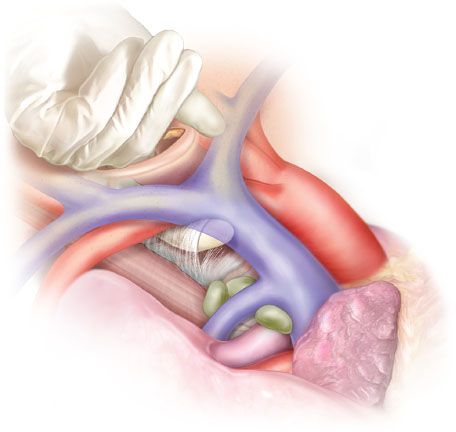
Figure 4.3 Pretracheal (perivisceral) fascia—anatomy and penetration of the fascia in the initial phase of mediastinoscopy.
The mediastinoscope is inserted while gently pulling the innominate artery ventrally with a retractor until the tip of the scope passes underneath. Dissection is performed by a blunt-tip suction monopolar or bipolar electrode by pushing the loose connective and fatty tissue away from trachea and occasionally coagulating the magnified and well-visible small vessels.
Visualizing the trachea and both main bronchi by dissecting under visual control and advancing the scope further along the trachea is essential for ascertaining the anatomical relationships and the actual position of the scope. The movement of the scope should neither follow a straight line nor should the scope be advanced in a drilling fashion the line is rather curved with a dorsal convexity so that the distal elevation of the dorsal spatula acts as a retractor pushing the fatty tissue or pulmonary artery ventrally and allowing further insertion of the scope (Fig. 4.4). Trachea serves as the essential landmark and in case of anatomical disorientation one should always retreat from terra incognita to identify the windpipe.
Cranial aspects of at least one or both main bronchi are usually easily visualized, thus yielding information about the approximate location of azygos vein and right pulmonary artery. Turning the distal end of the scope laterally enables dissection in paratracheal regions. On the right, the azygos vein arching over the right main bronchus enters the superior cava vein on its dorsal aspect. Sometimes transparent mediastinal pleura and the inflated lung parenchyma can be seen retrocavally in thin patients.
The left main pulmonary artery continues along the anterior, superior, and later the dorsal aspect of the left main bronchus. The position of the aortic arch prevents the scope from following the artery further. The distal course of the left recurrent laryngeal nerve curving laterally and anteriorly along the circumference of the aortic arch is identifiable. Ventrally from the tracheal bifurcation the relatively long intra- and extrapericardial course of the right main pulmonary artery can be observed and followed distally to the origin of anterior trunk (Boyden) and even further on its dorsal aspect between the main RPA and right main and intermediate bronchus. The right main pulmonary artery is usually retracted by the scope toward sternum when dissecting in the subcarinal area after the perivisceral fascia has been penetrated.
After advancing the 19-cm scope further caudally beyond the subcarinal nodes the esophagus may be visualized depending on its position subcarinally in the midline or left to it (dorsal to the left main bronchus). However, esophagus may also be vulnerable to inadvertent biopsy dorsally paratracheally on both sides.
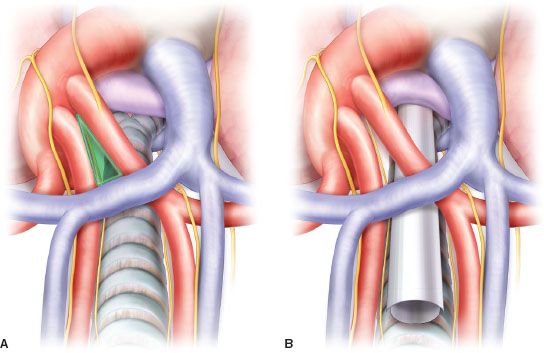
Figure 4.4 Superior view of mediastinal anatomy (A) and position of the mediastinoscope during dissection of the subcarinal zone, with right main pulmonary artery retracted by the superior blade (B). Note the “innominate triangle” for entry into aortopulmonary window.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


